Introduction
Tuberculosis (TB) is still endemic in South Africa.1 International and domestic literature confirms human immunodeficiency virus (HIV) to be a persistent driver of the presence of this ancient infection.1,2 Secondly, HIV co-infection has been shown to be associated with extra-pulmonary manifestations of TB, usually in individuals with previous or concurrent pulmonary involvement.3 The musculoskeletal system also bears the brunt of systemic tuberculosis and with an evident predilection for the spine and large joints.4 The hip and knee joints are the two commonly affected large extra-spinal joints.4,5
Making a diagnosis of musculoskeletal TB in both endemic and non-endemic regions of the world still requires a systematic clinical approach due to its varied presentation.3 With history taking, clinical examination, and an elaborative use of laboratory investigation a diagnosis is confirmed.3–5 One laboratory test that has been highly recommended in recent years for the diagnosis of osteoarticular tuberculosis has been the use of real-time polymerase chain reaction (PCR).1–7 However, in endemic countries such as South Africa and India the diagnosis of pulmonary TB has been made on clinical grounds for years now.2,4,5,8 Due to the cost of PCR and the fact that TB is endemic in our community we proposed the use of PCR in making a diagnosis of osteoarticular TB to be a costly exercise. Rather we propose that clinicians should rely on sound history taking, thorough clinical examination, and the use of basic cheap laboratory and pathological tests for the diagnosis of osteoarticular TB, especially in typical cases with suggestive radiological appearance (see Figure 1.)
Materials & Methods
This was a retrospective study at a single orthopaedic unit. The Wits Human (Medical) Ethics Research (HREC) granted clearance, certificate number M200750. The study only commenced after approval by the Chief Executive Officer. Admission notes and surgical and histopathological records of patients of all ages previously treated for confirmed extremity osteoarticular TB over a 5-year period were perused. Patient demographics, clinical presentation, and laboratory results were collected for analysis. All patients underwent surgical arthrotomy or incisional biopsy for synovial fluid and tissue samples microbiological and histopathological workup. Fluid samples underwent microscopy for Acid fast Bacilli (Figure 2), culture growths, and sensitivity analysis with additional samples administrated for GXP (PCR) for identification of the tuberculum and its sensitivity to Rifampicin. For histological assessment, the Ziehl-Neelson stain was used to assess for chronic necrotising granulomatous inflammation (Figure 3). All patients were treated for osteoarticular TB with 12 months of chemotherapy after diagnosis. This is the standard of care in our clinical unit coupled with supportive multi-disciplinary team involvement. At data analysis, some patients had not had their definitive surgical procedures.
Results
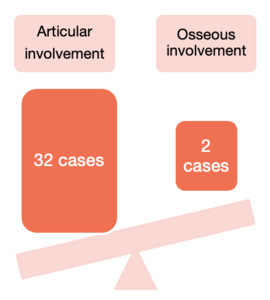
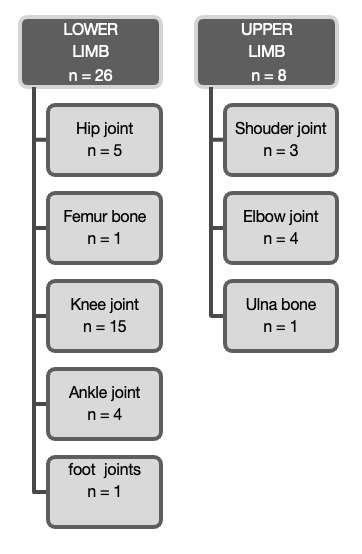
A total of 34 patients met the criteria for inclusion in our study. There were 17 females (50%) and 17 males (50%) in our audit all of whom were of African descent. The average age was 37.97 years (range 2 ‒ 64). The adult group (40 ‒ 60 years) accounted for slightly more cases (44%) followed closely at 41% by the young adult (40 ‒ 60 years) group, the groups are displayed in Table 1. We had one case of a two-year-old with TB osteomyelitis of the ulna. The lower limbs were affected in 76% (n = 26) of cases with the other eight accounted for by upper limb cases (see Figure 4). Moreover, Figure 5., shows that there was a clear predilection for articular involvement with 94% of cases affecting joints with just two cases (6%) localized to a distinct bone in the form of TB osteomyelitis. The two osseous cases in our study presented with osteomyelitis of the ulna and femur bone respectively. The knee joint was the most affected joint in our cohort with 15 out of the 32 joints being affected somehow (see Figure 4). The left knee and right knees were almost equally affected with a left to right knee ratio of 1:1.1.
Acid-fast bacilli (AFB) were positive in 32% (11 of 34) of the cases. Tuberculosis culture was positive for acid-fast bacilli in 29% (7 of 24 ) of cases. The histological diagnoses of necrotising granulomatous inflammation were diagnostic for 21 of 32 (66%) of the cases, which was only slightly superior to the 63% diagnostic yield of Real-time PCR for TB in our cohort. Unfortunately, PCR was performed only in 24 cases in our study due to cost and the fact that we get new doctors regularly who may not be familiar with our unit protocols. Sensitivity for Rifampicin was recorded only on 14 samples and fortunately, only one case (7%) was resistant to the drug rifampicin. Two PCR samples were inconclusive for sensitivity testing and therefore they were excluded from this analysis. Even with widespread counseling for HIV testing, only 22 patients tested for HIV or at least volunteered their HIV status. From this cohort we found 50% (11 of 22 patients) to be reactive for HIV. The presence of AFB on microscopy and the successful culture of the mycobacterium were compared. The sensitivity of a diagnostic histological sample versus a positive PCR result was analysed using the Chi-squared test. The yield rates between histology and PCR, however, were not statistically significant 66% versus 63%, respectively, with p-value> 0.05. Stata 17 software was used for analysis.
Discussion
Tuberculosis is still a concern worldwide.1,2,5 In the third world, however, it is endemic with India accounting for around 20% of the total worldwide TB burden.8 South Africa has the largest burden of HIV and TB co-infection in the world associated with a high burden of extra-pulmonary TB presentation.1,2 Our study found a 50% prevalence of HIV co-infection in osteoarticular TB cases that volunteered and/or agreed to HIV testing. Padayatchi et al. reported that 60% of their study population have HIV co-infection, a figure slightly higher than ours.2 The latter discrepancy could be due to our small study sample. Consistently low study samples were reported by Firth et al. (also from our Institution) looking at extra-spinal osteoarticular TB in 19 paediatric cases over a 16 years year period.9 Our study had one paediatric case out of 34 cases over 5 years. Studies with large sample sizes over a short duration were reported in China and Thailand with 43 and 99 osteoarticular TB cases recorded in just two years respectively, this is possibly due to a large population and ailing sanitation measures, respectively.5,10 In one notable review it was concluded the majority of the studies in the First World with large study cohort samples seemed to have biased inclusion of immigrant individuals from the Third World in those studies potentially skewing the results.10 In endemic areas such as India and South Africa the diagnosis of osteoarticular TB has always been made on clinical grounds, i.e. based on adequate history taking, clinical examination, and/or typical radiological changes.11,12 Naturally, high-index cases would then be treated empirically based on a clinical impression suggestive of osteoarticular TB diagnosis.8 However, this practice has lost favour due to an upsurge in cases of drug-resistant TB, therefore, fostering a need for drug sensitivity analysis to be determined before initiation of any anti-TB chemotherapy.8
TB infection in the skeletal system is usually paucibacillary, which poses a challenge to early diagnosis and treatment.8 Adequate and appropriate specimen collection and ideal transport media are critical for improved yield from MC&S, histological and PCR tests. The traditional diagnostic yield from microscopy for TB on synovial fluid has been diagnostic in a low 25% of cases, a figure further worsened in patients with HIV co-infection.5,7,8,10,13,14 A positive TB culture also has a relatively low yield due to the facultative nature of the mycobacterium with successful isolation of the tuberculum in 50 to 80% of cases only, however in our current study the isolation from culture was at a low 29% and we attribute this to the lack of Lowenstein-Jensen (LJ) medium at our centre and delays in specimen analysis.5,8,13 Reliance on MC&S is lowered due to prolonged incubation periods (3 to 4 weeks) and delays in the determination of sensitivity to anti-TB chemotherapy.8 PCR for TB diagnosis has gained popularity firstly for its rapid yield of results, usually within hours in well-organized centres.7,8,10,14 Secondly the sensitivity to Rifampicin is determined relatively fast, versus in MC&S.10 For synovial fluid in osteoarticular TB, PCR sensitivity for diagnosis was reported at (63%) by Aggarwal et al.11 The latter figure is similar to the one in our study (63%) it is well within a range from 32 to 78% that is reported in the literature.7,8 There has been a reported further improvement with a modified version of PCR namely, the Nested type. With Nested PCR, a sensitivity of up to 94% for the diagnosis of osteoarticular TB has been reported.8
In our study, the yield for TB on histology of biopsied tissue was 66%, a figure slightly higher than 47% reported in a study by Arathi et. al.5 However, in another study by Enache histology was diagnostic in 95% (18 out of 19), this high value could be due to sample size and pathologist’s experience.15 Histology for mycobacterium is usually a go-to for cases where MC&S results are inconclusive. However, in endemic areas, most clinicians again will make a diagnosis of TB based on the conclusion of “necrotising granulomatous inflammation”, even in the absence of actual mycobacterium bacilli. Unfortunately, histology diagnosed “necrotising granulomatous inflammation” only in two-thirds of our cases. This means that over a third of cases could be inconclusive cases if no further tests are done. Fortunately, Erdem et al. in their histological tissue analysis found histology for TB to be diagnostic in 90% of their cases while Jain et al. had a 100% diagnosis on histological analysis.13,16 The discrepancy with our cases can be due to a low study population and a heavy reliance on pathologists in training for tissue sample analysis.
Conclusion
Proper clinical assessment, diagnosis, and management of osteoarticular TB are important. A high index of clinical suspicion is required for prompt diagnosis. In our local setting off a high prevalence of HIV co-infection with TB infection as well as poor outcomes of TB infection, early diagnosis and treatment of both conditions is critical. In numerous studies published in the literature, PCR is shown to have a higher sensitivity for osteoarticular TB diagnosis. Our study showed a lower PCR diagnostic yield and rifampicin sensitivity profile similar to traditional histological analysis and MC&S, respectively. However, the other benefits of PCR cannot be ignored, especially the quicker result turnaround time. The downside of PCR still seems to be its monetary cost while histology and culture are operator-dependent and time-consuming. With all being said we advocate for MC&S and histological analysis by experienced pathologists in resource-stricken center like ours.
Acknowledgements
To both Prof. M.T. Ramokgopa and Dr. M. Jingo for the encouragement.
Author contributions
Dr. Marule Paul Kgagudi: substantial contributions to the conception and design of the work, and the acquisition, analysis, and interpretation of data for the work, drafting the work, and revising it critically for important intellectual content.
Dr. Tiego Hlapolosa and Prof. Mmampaptla Thomas Ramokgopa: substantial contributions to the conception and design of the work, and the acquisition, analysis, and interpretation of data for the work and revising it critically for important intellectual content.
Dr. Maxwell Jingo: substantial contributions to scientific input and critically important intellectual content revision.
Dr. Eunice Van Den Berg: pathology review and image library supplementation.
Funding
The study was self-funded by the authors.
Conflict of interest
No conflict of interest or disclosures to be made by all authors.