Background
In 2019, a total of 238,697 arthroplasties were performed in the United Kingdom (UK).1 Despite advances in surgical techniques, periprosthetic joint infections (PJI) remain an ongoing concern. The incidence of PJI is estimated to be between 1-3%.2 The anticipated increase in PJIs underscores their significant impact. The financial burden on healthcare systems, such as the United Kingdom’s National Health Service (UK NHS), is substantial, with the cost of treating PJIs estimated to reach £21,937 per case.3 This figure does not take into account the extended use of antibiotics, functional impairment, or the complications that may arise as a result and so the true cost may be substantially greater.
The mortality rate associated with PJIs is a significant concern. At the one-year mark, the mortality rate post two stage revision for infected total hip arthroplasties (THA) was 4.22%, gradually increasing by an average of 1.93% per year to a 21.12% mortality rate at the five-year milestone.4
The difficulty in diagnosing periprosthetic joint infections (PJIs) is well-documented in the literature.5,6 When the cardinal signs of inflammation are present, it becomes easier to recognize PJIs.5 However, there is a risk that indolent and less virulent infections may be missed, leading to potential misdiagnoses.6 This can be particularly detrimental when patients with undetected infections are treated for aseptic loosening instead of an infected prosthesis.7
As it stands the main difficulty in PJI treatment is early diagnosis. The Musculoskeletal Infection Society (MSIS) criteria 2018 provides an evidence-based approach for diagnosing PJIs with high levels of sensitivity (97.7%) and specificity (99.5%).8 Several other diagnostic criteria have been proposed for diagnosing PJIs, but there is no one test which can completely confirm the diagnosis of a PJI.8–12 Some features such as a sinus tract, see Figure 1, are pathognomic for a PJI.7,8,13,14 However, this is not always present. In our experience the presentation is highly variable and dependent on a number of factors including the virulence of the organism and the physiology of the host.
A common symptom across all patients is the presence of pain. In a study conducted by Tsaras et al, 7,375 arthroplasty implants were analysed to determine the incidence of PJIs.15 In those that were identified with a PJI, pain or tenderness was reported in 94.6% of the cases (n=70). Other symptoms included swelling or effusion in 48.6% (n=35), redness or erythema in 38.9% (n=28), warmth in 34.2% (n=25), fever in 36.5% (n=27), and purulent drainage or sinus tract in 39.7% (n=29).15 The variability in clinical presentation is likely influenced by the timing of implantation, as traditional symptoms of infection tend to manifest more acutely following the surgical procedure or bacteraemia. Hence, a thorough clinical history and physical examination are crucial components in the diagnostic process, but they should not be the sole factors relied upon for diagnosis. The review summarises the current evidence-base for emerging and novel biomarkers of PJI.
Serum Biomarkers
C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)
When a suspicion of PJI arises, most clinicians utilize serum biomarkers as an initial screening tool to assess the likelihood of infection. CRP and ESR are two widely available and sensitive blood tests that serve as essential diagnostic aids in screening for potential PJIs. According to the recommendations of the 2018 International Consensus Meeting on PJI, they may be used for diagnosis.8 During the early phase of an infection, the presence of cardinal signs of inflammation is often accompanied by elevated levels of CRP & ESR.
Consequently, increased CRP and ESR values can help to confirm the presence of PJI. The sensitivity of ESR is 42–94% and its reported specificity ranges from 33–87% in the literature.16 The sensitivity of CRP is 74–94% and its specificity varies between 20% and 100%.17 Furthermore, it has been shown that the CRP level normalises at approximately three weeks, while the ESR level may be elevated for up to one year after total hip and knee arthroplasty.18–21
In late or chronic cases of PJIs, CRP has been shown to have limited utility due to its low sensitivity and specificity. Fernández-Sampedro et al analysed a total of 498 patients, including 77 late PJIs. In these late PJIs, the sensitivity of CRP was only 62.3%, which is significantly lower compared to other diagnostic methods.22 This indicates that relying solely on CRP levels in late or chronic cases may lead to high false-negative rates, potentially delaying appropriate diagnosis and treatment. Therefore, it is important to consider other diagnostic methods when evaluating patients with suspected delayed and late-stage PJIs, rather than relying solely on CRP levels.
Furthermore, the microbiological agent responsible for the infection can impact CRP levels in PJIs. Bacterial agents with high virulence factors, such as Staphylococcus aureus, have been shown to produce higher median CRP levels compared to low virulence organisms, such as Coagulase-negative Staphylococci or Propionibacterium acnes (also known as Cutibacterium acnes).23 Akgun et al conducted a retrospective analysis of 215 patients, examining their presentation and investigations related to PJIs.23 Their findings revealed that patients with S. aureus infections had a median serum CRP level of 194 mg/l, while those with Coagulase negative Staphylococci and Propionibacterium species spp. infections had significantly lower median CRP levels of 12.2 mg/l and 5.4 mg/l, respectively (p < 0.001).23 Additionally, the study found that 81.3% (n=59) of cultures were positive for S. aureus, compared to 56.1% (n=41) for Staphylococcus epidermidis and 34.6% (n=9) for Propionibacterium species spp.23 This difference in CRP levels and culture positivity rates highlights the influence of the microbiological agent on CRP levels in PJIs.22,23
Low virulence PJI producing relatively modest inflammatory marker elevation, as described above, makes defining a robust biochemical marker threshold challenging. Over the past decade, several authors have suggested lowering the thresholds for CRP and ESR in PJI, with Bingham et al. finding optimal diagnostic cut-offs for CRP and ESR of 5mg/L and 10mm/h.24–26However, some research suggests that these thresholds may vary between both acute vs chronic PJI and by affected joint.27,28 Multiple studies have presented higher CRP thresholds in acute PJI, ranging from between 23.5 mg/L to 38.9 mg/L..27–29Additionally, Alijanipour et al. found that CRP was higher in knees affected by PJI than in hips affected by PJI.27They therefore suggested that a lower CRP threshold of 13.5 mg/L should be used in hips suspected of acute PJI, compared to 23.5 mg/L in knees, whilst similar ESR thresholds could be applied (48.5 mm/hr in hips compared to 46.5 mm/hr in knees).27Similarly, Uvodich et al. proposed a higher CRP threshold of 34 mg/L for total knee arthroplasties (TKAs) suspected of PJI at 6-12 weeks.30
Finally, another limiting factor with the use of CRP levels is that it is non-specific biomarker that can be elevated with other conditions, therefore, may be of limited value in someone with malignancy or recent trauma or surgery.22,23
Interleukin-6 (IL-6) is a cytokine that is released in the presence of bacterial infection or tissue damage. It has shown some utility in the diagnosis of chronic PJI. In a study conducted by Randau et al, serum IL-6 levels were analysed in 120 patients admitted for revision arthroplasty. The results demonstrated that IL-6, as a serum biomarker, offered a specificity of 88.3% in distinguishing between PJI and aseptic loosening.31 A 2010 meta-analysis showed superiority of IL-6 over CRP and ESR in diagnosing PJI, with a sensitivity of 97% and specificity of 91%.32 With the combined use of IL-6 and serum CRP, Elgeidi et al. reported a sensitivity of 100%, specificity of 99% and accuracy of 98%.33 Nonetheless, as pointed out by Randau et al, there is a lack of consensus regarding the definitive cut-off values that signify infection.31 Moreover, it’s worth noting that this test is relatively costly and not commonly utilized, thus limiting its practicality and accessibility in a wider context.31,33
D-dimer represents another viable biomarker. It is a product of fibrin-clot dissolution by plasmin. The increased fibrinolytic activity associated with infections results in increased D-dimer levels. In a prospective study by Shahi et al involving 245 patients admitted for revision due to PJI, aseptic failure, or primary arthroplasty, the levels of ESR, CRP, and D-dimers were compared.9 The results indicated significantly elevated D-dimer levels in PJI patients versus the primary and aseptic loosening groups.9 Moreover, D-dimer demonstrated a higher specificity (93%) and sensitivity (89%) compared to both ESR and CRP, which reported sensitivities of 73% and 79%, and specificities of 78% and 80%, respectively.9 Xu et al. published a meta-analysis reporting 89% sensitivity and 76% specificity of serum D-dimer for PJI.34 However, other studies suggest that there is no discernible superiority among these markers and that when used alone, D-dimer is insufficient to diagnose PJI.35,36 Particularly, Wixted et al. have questioned the diagnostic utility of D-dimer for acute PJI, finding no significant difference in D-dimer values (within 90 days post-operatively) between patients with acute PJI and a control group.37 It is worth noting that several studies have reported increased diagnostic accuracy of serum D-dimer compared to plasma D-dimer, so one should be cognisant of the method of sampling and heterogeneity this can cause if not accounted for.38,39 One must also exercise caution as raised D-dimer can occur as part of a normal post-operative course and in a number of other conditions including acute thrombotic events.36,39
Pro-calcitonin (PCT) has gained recent attention as a potential marker, primarily due to its utility in identifying bacterial infections. PCT is normally produced by the thyroid gland whereas in infectious conditions it is produced by macrophages and liver-derived monocytic cells. PCT efficacy in pinpointing PJIs has been inconsistent in the literature. A study by Guerrero et al revealed that serum PCT exhibits a broad spectrum of sensitivity (33-90%) and specificity (27-98%).40 This considerable variation suggests potential limitations in its reliability as a marker. Furthermore, a study conducted by Yuan et al indicated no significant difference between CRP and PCT in their diagnostic accuracy for PJI.41
Serum Neutrophil, Serum Neutrophil Lymphocyte Ratio and Number of Serum Platelets/ Mean Platelet size have been proposed as novel diagnostic serum biomarkers and are currently being studied as new diagnostic tools. In particular, early studies of serum neutrophil lymphocyte ratio are showing promising potential in PJI.42
Imaging
According to the MSIS 2018 criteria, diagnosis of PJI can be made using “major criteria” that include clinical assessment revealing a sinus communicating with the prosthesis or if the pathogen is isolated by culture from two separate samples from the affected joint.8 However, additional “minor criteria” obtained from serum tests, synovial samples, and histological analysis are usually required to confirm the diagnosis in practice. In our experience, adjuvant imaging may also be helpful in diagnosing PJI and planning further treatment.
Plain Radiography is a fundamental and widely used initial imaging method. Despite limited sensitivity and specificity for PJIs,43 it is essential for differential diagnosis, especially in excluding fractures or tumours. Depending on the location of the suspected PJI, radiographic findings vary in their ability to detect infection.
Zajonz et al. conducted a thorough study with 320 patients diagnosed with PJI, which included an in-depth review of diagnostic evaluations.44 This encompassed an analysis of 119 hip and 118 knee radiographs.44 Radiographic evidence of infection was demonstrated in 61% of hip cases, while this figure was 29% for knee cases.44 The study underlines that the anatomical location of PJI has distinct clinical and radiographic presentations. The authors speculate that hip PJIs take longer to manifest, leading to more pronounced radiographic signs of infection.10,44,45 Radiographs primarily focus on identifying radiolucency and osteolysis as key signs, and rapid migration of the prosthesis, exceeding 2mm within a 6-12 month period, has also been established as surrogate markers of infection.46,47
However, it is crucial to recognize that these features may be absent in approximately 50% of cases.48 Moreover, they are not pathognomonic for PJI and can be associated with aseptic loosening and other pathologies.7,49,50 Stumpe et al. conducted an analysis on conventional radiographic findings, demonstrating that these findings have a sensitivity in the range of 78-89% and a specificity between 50-65%.47 Augmenting radiography with the integration of fistulography and arthrography may enhance the probability of detection.51,52 Other findings such as periarticular ossification, can be strong indications for infections but not necessarily pathagnomic of PJIs.53
Computed Tomography (CT) scans afford enhanced imaging of the soft tissues surrounding an arthroplasty, which can be pivotal in indicating the presence of a collection or PJI, as elucidated by Chang et al.54 Furthermore, CT imaging is adept at detecting pathologies that might simulate the presentation of PJIs, such as psoas abscess, as documented in a study by Atif et al.55 Cyteval et al. undertook a prospective investigation in which 65 patients, manifesting clinical indications suggestive of infection, were evaluated.56 Subsequently, these patients underwent revision arthroplasty after a span of one month, during which microbiological cultures were acquired to ascertain the presence of PJI. The investigation revealed that the sensitivity of diagnosing infection based on soft-tissue CT findings fluctuated between 83% to 100%, and the specificity oscillated between 87% to 96% in patients with hip prostheses.56
Nevertheless, CT scans exhibit inherent limitations, particularly in their inability to discriminate between aseptic loosening and PJIs. Additionally, the presence of metallic components in prostheses generates artifacts that can hinder the accurate interpretation of images. Notably, in recent years, the application of digital tomosynthesis has shown promise in enhancing the detection of osteolysis by mitigating the interference of metallic artifacts.57 However, as digital tomosynthesis is not extensively available, further investigation is required to fully ascertain its utility.
There is a general consensus in the medical community that plain radiographs have limited diagnostic utility in ruling out infection. However, in cases with high clinical suspicion of infection, they are considered useful in assessing the extent of bone involvement and soft tissue infection.
Magnetic Resonance Imaging (MRI) is an advanced imaging modality that generates detailed images of soft tissues. It is also effective in detecting alterations in bone structures, assessing changes in bone marrow, and analysing fluid content and distribution in and around anatomical structures. However, artifacts, particularly in cases with metallic prostheses, have been a concern as they can hinder image interpretation. The introduction of Metal Artifact Reduction Sequences (MARS) has addressed this issue, leading to an improvement in diagnostic yield.58 However, it does not eliminate it completely. Despite this, they have demonstrated use in identifying infection in a select group of patients.
It has been shown that metal artefact reduction sequences in magnetic resonance imaging (MARS MRI) may help to distinguish between PJI and aseptic failure.59 Synovial layering and muscle oedema are significant features of periprosthetic joint infection, with sensitivities of 100% and specificities of 63.0-75.0%.59 Granulomatous synovitis is a significant feature of aseptic failure, with 90.0% sensitivity and 57.0% specificity.59 The MARS MRI is also a useful diagnostic tool for diagnosing adverse reaction to metallic debris (ARMDs).60 It is important to remain vigilant for soft tissue sarcomas presenting as pseudotumours around joint replacements and obtaining biopsies if needed to confirm the diagnosis.61
Li et al analysed 108 MRI scans of patients following a TKA.62 These patients underwent a revision surgery, either as an index revision TKA (first revision) or a revision of a previously revised TKA.62 The study found that MRI could identify signs of infection with varying degrees of sensitivity and specificity depending on whether it was an index or revision TKA.62 For index TKAs, the sensitivity ranged from 84.6% to 92.3%, and the specificity was 100%.62 In contrast, for revision TKAs, the sensitivity was between 40% and 60%, while specificity ranged from 92% to 96%.62 These findings suggest that MRI can be a useful diagnostic tool in ruling out PJIs in those who have undergone total knee arthroplasty, particularly in cases of index revision TKAs.
In the context of PJIs, nuclear medicine has provided useful diagnostic insights. Techniques such as triple phase bone scintigraphy (TPBS) using 99mTc labelled diphosphonates and fluorodeoxyglucose (FDG) positron emission tomography (PET) are often deployed. In particular, WBCs labelled with 99mTc-hexamethylene-propyleneamine oxime (HMPAO) or Indium oxine, represent the gold standard imaging technique for diagnosing PJI with high sensitivity and specificity.63,64 It is the only imaging modality to be considered in the European bone and joint infection society criteria for diagnosing PJI.14 These imaging modalities are most useful in the diagnosis of chronic PJI, which is defined as infection more than three to six weeks after surgery.
TPBS is a test that is widely available and often used as an initial test to diagnose PJIs. The technique entails the utilization of technetium-99m (99mTc) labelled diphosphonates, which exhibit preferential aggregation in regions characterized by elevated blood flow and osteoblastic activity. TPBS is systematically executed in three temporally distinct phases: the perfusion phase, the blood pool phase, and the osteotropic phase. The perfusion phase is initiated to evaluate the perfusion status of the targeted region. Subsequently, the blood pool phase commences following the injection of the radionuclide dye and is designed to assess vascularity within a timeframe of 3-5 minutes post-injection. The osteotropic phase constitutes the final stage and is dedicated to the assessment of bone turnover. A concurrent uptake across all three phases is indicative of inflammation, rendering it a surrogate marker for PJIs.65
Ouyang et al. advocate for the incorporation of Triple Phase Bone Scintigraphy (TPBS) as a preliminary screening modality for Prosthetic Joint Infections (PJIs).66 In their rigorous investigation, a meta-analysis was conducted encompassing data from 702 patients across 20 studies, with the objective of appraising the diagnostic accuracy of TPBS.66 The study discerned that the pooled sensitivity and specificity for TPBS in the detection of PJIs were 83% and 73%, respectively.66 A more granular examination of the data revealed that the anatomical location of the prosthesis was a significant determinant in the diagnostic accuracy.66 Specifically, hip prostheses manifested higher sensitivity and specificity values, at 81% and 78% respectively, compared to knee prostheses, which exhibited sensitivity and specificity values of 75% and 55%, respectively.66 However, increased uptake has been observed in other cases such as neoplasms and aseptic loosening.64 Therefore, it has potential for a screen test.64,66
An alternate modality employs leucocytes. In this technique, WBCs are extracted, labelled with HMPAO, and reinfused into the subject. Post radiopharmaceutical injection, three temporal phases ensue: early (30 minutes to 1 hour), delayed (2-4 hours), and late (20-24 hours).63 Interpretative analysis is conducted during the intervals between these phases. While this technique offers diagnostic utility, it is associated with complexity, high costs, and extensive time consumption. Notably, the results remain unconfounded by other inflammatory processes.67 This is combined with other types of imaging.
The WBCs labelling technique with HMPAO is considered the gold standard in imaging for PJIs. Love et al. conducted an analysis involving 150 patients who underwent leucocyte scanning, revealing a sensitivity of 96%, a specificity of 87%, and an overall diagnostic accuracy of 91%.68 These findings are substantiated by Reinartz et al., who reported comparable sensitivity values of 91% for hip prostheses and 84% for knee prostheses.69 However, as highlighted above, this is invasive for patients, it requires multiple visits and long stays and is costly. As a result, Reinartz advocates for the use of PET-FDG69 as they stipulate that it obtains a similar diagnostic performance compared to WBC scans but they are less invasive and time consuming.69 PET-FDG is a well-established tool in oncological imaging and has been shown to identify infection and inflammation.70,71 In infection and inflammation, the increased glycolytic activity in neutrophils and activated macrophages leads to FDG uptake.72,73
Nonetheless, the diagnostic accuracy of these modalities remains a subject of debate. Verberne et al. conducted a pooled meta-analysis and reported an initial sensitivity of 70% and specificity of 84% for PET-FDG74 in contrast to 88% and 77% respectively for WBC scans.74 Upon exclusion of high bias studies, the sensitivity of FDG-PET escalated to 91%, which was statistically indistinguishable from that of WBC scans (p=0.39).74 However, Verberne et al. contend that this increment in sensitivity does not warrant the financial investment entailed in employing PET-FDG.74
Advancements in hybrid imaging and integration of imaging modalities have demonstrated a propensity for enhancing diagnostic yields,69,74 marking this as a domain in evolution. Recently, Nie et al. endeavoured to harness artificial intelligence (AI) for PJI diagnosis using TPBS.75 A retrospective analysis was performed on 449 patients with established diagnoses, which were utilized to architect a diagnostic framework.75 The study exhibited a diagnostic accuracy of 86.48% for knee PJIs and 86.33% for hip PJIs, juxtaposed with established nuclear medicine specialists.75 The prospective implications of AI adoption in the realm of PJI diagnostics warrant further investigation.75
Synovial fluid analysis
Serum biomarkers are not necessarily increased in low-grade virulence infections which has led to the emergence of synovial fluid biomarkers as an imperative test in the diagnostic process, following the initial stages of blood assessments and imaging tests. The investigative analysis encompasses cytological evaluation of the fluid, Gram staining techniques, and subsequent culture procedures. Additionally, the identification and measurement of specific biological markers contribute significantly towards augmenting the diagnostic accuracy.
In the context of synovial fluid analysis, cytology plays a pivotal role. This method encompasses the enumeration of total leukocytes along with the assessment of the polymorphonuclear neutrophil (PMN) percentage. Various cut-off settings have been used with various sensitivity and specificity values. The original synovial leukocyte count cut-off value of >10,000 cells/μL proposed by the International Consensus Meeting has been shown to have low sensitivity by multiple authors, in both acute and chronic PJIs.25,29 In acute PJI, Xu et al. found that a synovial leukocyte count cut-off of >10,000 cell/μL only had a sensitivity of 59.6%.25 Sukhonthamarn et al. subsequently demonstrated that the optimal synovial leukocyte count threshold was 6,130 cells/μL at 90 days post-operatively, producing 91% sensitivity and 83% specificity.29 For chronic PJIs, both Parvizi et al. and McNally et al. suggested an even lower demarcation value of >3000 leukocytes/µL, with PMNs percentage of 65-80%.8,14 Della Valle et al demonstrated that this cut off has a sensitivity of 100%, specificity of 98%, and accuracy of 99% in chronic PJIs.76
Nevertheless, cytological assessments can be influenced by a variety of host-related factors, one notable example being the timing of sample collection. This observation is supported by a study conducted by Bedair et al. where patients, not afflicted with prosthetic joint infection (PJI), manifested counts exceeding 4000 cells/µL in the post-operative phase.11 Intriguingly, the specific microbiological agent can also influence the measurements. As per the study conducted by Trampuz et al. synovial leukocyte counts were found to be higher in instances of Staphylococcus aureus infections as compared to infections characterized by lower virulence such as Coagulase-negative Staphylococci.77 It is worth noting that there are some limitations with this method which include the need for a high-quality synovial fluid sample since blood admixture with joint fluid makes quantitative and qualitative examination difficult.78 Furthermore, one must consider whether a patient has had antibiotics before aspiration when interpreting synovial fluid results. Massey et al. showed that the synovial fluid leukocyte count diagnostic cut-off dropped from 33,000 cells/μL to 16,000 cells/μL in patients who received antibiotics before aspiration for native joint septic arthritis.79 Whilst this study is in native joint septic arthritis, it is worth being aware of as its results may translate to PJI.
Alpha-Defensin (AD) is an antimicrobial peptide secreted by synovium neutrophils in reaction to infection and targets the cell membrane of the infecting agent. It has been reported to exhibit a sensitivity range of 96-100% and a specificity exceeding 90%.12,80–83 Significantly, this biomarker’s diagnostic accuracy is not compromised by the administration of antibiotics84 and it possesses the capability to identify a broad spectrum of microbial agents exhibiting a diverse range of virulence.85 A recent large-scale meta-analysis reported laboratory based synovial AD and synovial calprotectin were the two best independent preoperative diagnostic tests for PJI.86 The assay is available in two configurations: a qualitative lateral flow test (LFT), which presents lower diagnostic accuracy, and a quantitative method, the enzyme-linked immunosorbent assay (ELISA). While the latter offers superior accuracy,87,88 it is not as widely accessible and requires a more labour-intensive process. It is our recommendation that a LFT be used perioperatively to confirm suspicion of a PJI. The use of AD prior to implantation of prostheses in cases of two-stage revisions, did not add any benefit according to Owens et al.89
The Leucocyte Esterase (LE) assay presents a swift diagnostic method to identify potential PJI. This qualitative analysis aims to detect the presence of LE within synovial fluid. This enzyme is characteristically released by neutrophils as a response mechanism to infectious stimuli.8,90 A positive (“+”) reading on the LE test may suggest the existence of an acute infection, while a double positive (“++”) serves as a threshold indicative of a chronic infection.90 Despite the notable advantages of this test, including cost-effectiveness, wide availability and rapidity, the interpretation of outcomes remains susceptible to observer bias.91 Electronic readers have been employed to mitigate the bias but their ability to do so remains to be seen.
Calprotectin, a recently identified biomarker, is a protein secreted by neutrophils and stimulates leucocyte migration as part of the inflammatory response. It is garnering increased attention and utilization in the medical field. Historically employed in the detection of inflammatory bowel diseases, its application in diagnosing PJIs is currently a burgeoning field of interest. Hantouly et al conducted a comprehensive meta-analysis comprising 618 subjects across eight studies looking to the diagnostic accuracy of calprotectin. They found a cumulative sensitivity and specificity of 92% and 93% respectively.92 A recent meta-analysis showed synovial Calprotectin is a promising biomarker of PJI diagnosis. The distinct advantages of this assay are its cost-effectiveness, wide accessibility, and prompt delivery of results.92 Nonetheless, the definitive value and impact of calprotectin as a diagnostic tool in this context remain under active research and exploration.92
Synovial CRP, the combined utilization of synovial C-reactive protein (CRP) and serum CRP has demonstrated superior diagnostic precision relative to the exclusive use of CRP. In a study conducted by Baker et al, an analysis was undertaken of 621 patients being evaluated for a revision arthroplasty due to potential PJI.93 Both serum and synovial CRP levels were examined, and the combination of the two resulted in an enhancement in diagnostic accuracy. They reported sensitivity as 74.6% and specificity of 98% with superiority to the serum CRP.93 Nevertheless, similar to serum CRP, the administration of antibiotics or the presence of other inflammatory or neoplastic conditions could influence the levels of CRP.
D-lactate, an additional biomarker applicable in the analysis of synovial fluid, is a metabolic by-product derived from bacterial activity, typically present within infected tissues.94 It has been recommended as a screening test by Karbysheva et al, who tested 224 patients with suspected PJI synovial fluid and found that it had a 92.4% sensitivity and 88.6% specificity.95 Given that this metabolite, D-lactate, mirrors bacterial activity, it is plausible that diminished bacterial activity correlates with decreased D-lactate concentrations, particularly in regions exhibiting biofilm presence.96 Moreover, the bacterial virulence factor also influences D-lactate levels, with non-virulent organisms tending to produce lower quantities of D-lactate in comparison to their virulent counterparts.95,97 The advantages of this approach encompass a reduced sample volume requirement for testing, expedited result delivery, and cost-efficiency.
Finally, one should be aware of the potential for an inadequate aspirate or ‘dry tap’. In these scenarios, one can re-position the needle under fluoroscopic guidance and gently manipulate the joint to encourage movement of synovial fluid within the joint. Both of these techniques have been documented in hip joint aspiration, particularly fluoroscopic visualisation of needle placement deep to the inferomedial femoral neck.98–100 If still no aspirate is returned, saline lavage and aspiration of the joint can be attempted. Ali et al. reported that there was no statistically significant difference in culture sensitivity between hips injected and not injected with saline prior to aspiration (83% vs 82% respectively).101 However, a more recent study by Heckman et al. suggests that culture sensitivity may be affected by saline lavage.102 Heckman et al. compared intra-operative synovial fluid samples before and after lavage with 20ml of saline and found that of ten positive pre-lavage fluid cultures, only six remained positive post-lavage.102 They also showed that although synovial white blood cell count was significantly lowered by saline lavage, %PMN remained similar post-lavage with a reasonable sensitivity of 75% (at a threshold of 80% PMN) for PJI.102 Furthermore, Christensen et al. found that patients with a dry tap had a higher rate of negative pre-operative cultures followed by positive intra-operative cultures compared to patient with successful aspirations (85.7% vs 41.1%, P = .047).103 The authors therefore suggested that surgeons should treat negative pre-operative cultures obtained after saline lavage with particular scrutiny.103
Microbiological and Histological analysis
The accurate identification of the causative microorganism is unequivocally a crucial aspect in the management of PJI. The conventional diagnostic approach has relied heavily on the cultivation of synovial fluid. However, recent evidence has demonstrated this method’s suboptimal diagnostic accuracy in chronic PJI cases. This phenomenon can be attributed to a multifaceted confluence of factors.
Primarily, bacteria associated with chronic PJI are typically characterized by a reduced virulence and correspondingly slow replication rate.104 This characteristic was exemplified in the study conducted by Schäfer et al, in which 284 patients with suspected PJI underwent culture tests with a 14-day incubation period, concurrently compared with histological analysis.104 Findings from the study indicated an overall detection rate of 73% via culture method alone.104 It was observed that less virulent organisms, including Propionibacterium acnes and Coagulase-negative Staphylococci, were identifiable within the 14-day timeframe.104 However, the more virulent Staphylococcus aureus was detectable considerably earlier, typically within the first week.104 This suggests a differential temporal pattern of bacterial growth, contingent upon the inherent virulence of the microorganisms involved.
Secondly, tissue and aspiration culture sensitivity have been shown to be significantly reduced by the use of pre-sampling antibiotics. As early as 1997, Barrack et al. demonstrated that pre-aspiration antibiotics reduced aspiration sensitivity from 75% to 41.6%.100 . Since then, Trampuz et al. have also reported the sensitivity of tissue culture decreasing from 60.8% to 45% if antibiotics were given in the two weeks before surgery105 Similarly, Malekzadeh et al. found that the odds of having a culture negative specimen increase 4.7-fold if the patient had received antibiotics within the 3 months prior to aspiration or surgery.106 It is therefore recommended that antibiotics are stopped a minimum of two weeks before aspiration, where clinically safe to do so.100,101,107
Furthermore, chronic PJIs are associated with an established biofilm on the prosthesis. Culturing techniques are designed for “free floating” or planktonic bacteria to be captured and cultured on nutrient mediums, however bacterium in biofilm are not metabolically as active as their planktonic counterparts, they are in the sessile state and therefore difficult to culture.108 In addition, the bacterium produces a complex matrix composed of polysaccharides, deoxyribonucleic acid (DNA) and proteins known as an extracellular matrix (ECM). This ECM also makes it difficult to obtain viable samples for culturing by trapping any planktonic bacteria preventing them being secreted into the synovial fluid.109 These biological differences have been reflected clinically in the necessity for extended culture incubation time up to 14 days in chronic PJI but not in acute PJI.110,111
The probability of achieving successful cultivation is enhanced by the disturbance of the ECM and biofilm, which can be accomplished through various modalities, including sonication or chemical interventions. Trampuz et al. provided empirical evidence of this when they analysed 24 prosthetic devices that underwent sonication post-removal, comparing their results to those obtained from standard tissue cultures.105 Their study exhibited an upsurge in sensitivity post-sonication, marked at 75%, a considerable increase compared to the 54% observed in standard cultures.112 Furthermore, sonication was also shown to enhance the detection of less virulent organisms.113 In their study of 145 PJIs, Bellova et al. not only corroborated the findings of Trampuz et al., but also reported an escalated frequency of Coagulase-negative Staphylococci, organisms traditionally difficult to culture.113
Nevertheless, the risk of contamination is an important factor to consider, as pointed out by Trampuz et al., necessitating careful handling.112 This was highlighted in a study by Park et al., wherein sterile femoral implants were inoculated, autoclaved, and then re-cultured post-sonication.114 The results showed growth of bacterial organisms that were not part of the original inoculation, underscoring the inherent risk of contamination.112,114
These methodologies primarily depend upon traditional microbiological techniques, but current trends are shifting towards molecular diagnostic procedures. Goswami et al. conducted a multi-centre analysis on 85 patients with culture-negative PJIs, implementing Next-Generation Sequencing (NGS) to ascertain the presence of organisms.115 Results revealed that bacteria were identifiable in 65.9% of patients with culture-negative PJIs using NGS.115 In a separate investigation, Kuo et al. demonstrated the superiority and time-efficiency of Matrix Assisted Laser Desorption/Ionization - Time of Flight (MALDI-TOF) relative to conventional swab techniques.116 Through examining 77 patients with PJIs, a comparative analysis of diagnosis times between MALDI-TOF and conventional swabs was conducted.116 The diagnostic precision of MALDI-TOF was determined to be 80%, a significant improvement over the 59% accuracy of swabs, with results produced earlier.116 Despite the increased accuracy and speed of these methods, there are accompanying drawbacks. These procedures are labour-intensive, expensive, and due to their heightened sensitivity, carry a substantial risk of contamination. Furthermore, their current capabilities do not extend to determining resistance profiles. Although they can detect the presence of resistance genes within the genotype, they lack the ability to confirm if these will manifest in the phenotype.
Histological examination has proven to be an indispensable tool in the field. Inagaki et al. conducted a comprehensive histological and microbiological analysis of 60 PJI specimens and 78 specimens from aseptic implant failures, utilizing a well-established guideline for PJI detection.117 Their findings indicate that a combined approach of histological and microbiological analyses yielded an accuracy rate of 98.6%. Furthermore, this approach was effective in accurately identifying aseptic failures.117
An additional advantage lies in the analysis of intraoperative histological findings for inflammatory cells in the event of a sample testing positive for microbial growth. This approach enables the determination of PJI-specific features within the sample, thereby providing corroborative evidence for the microbial findings.
Scoring Models
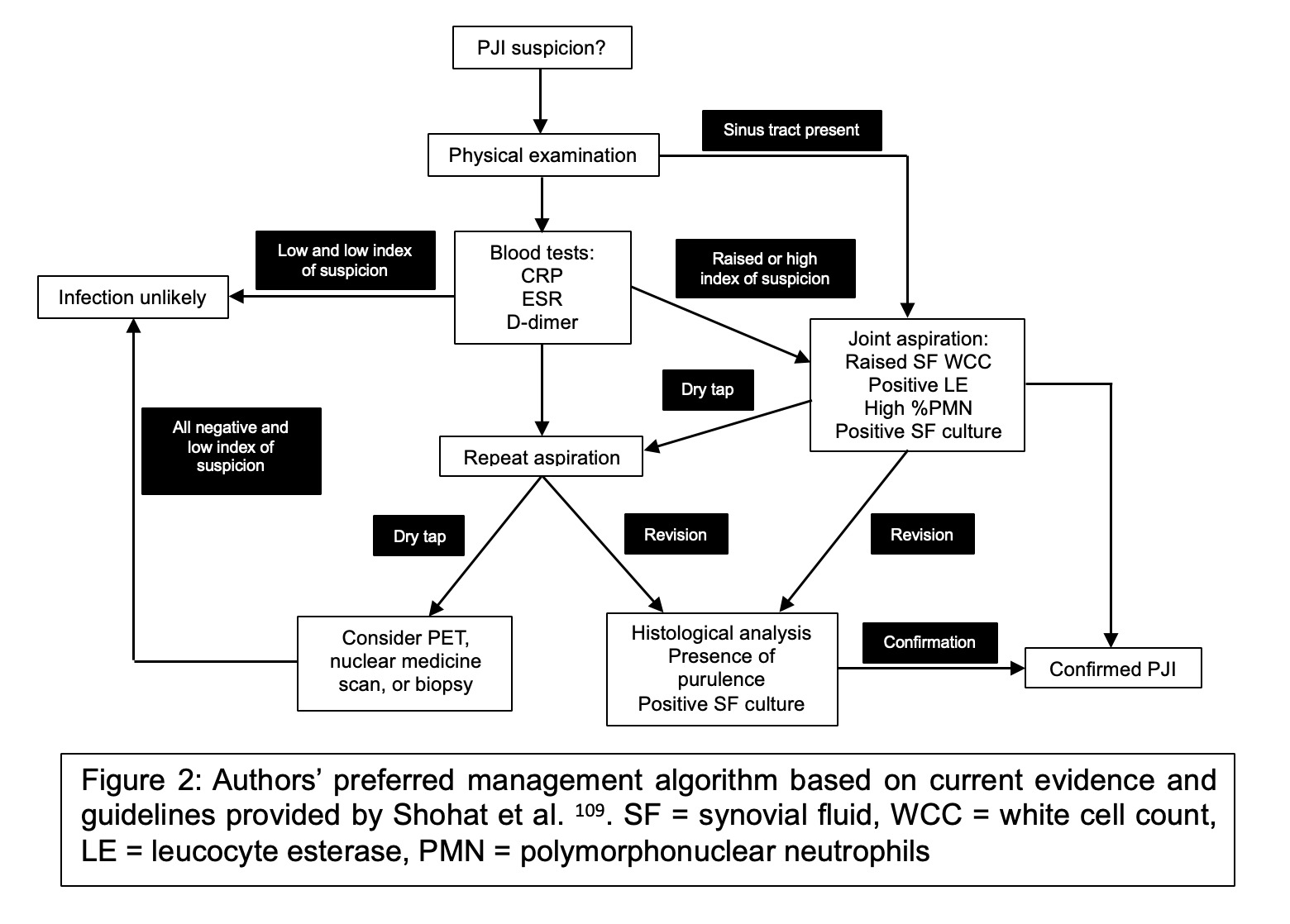
As previously discussed, there is no definitive “gold standard” diagnostic test for PJIs. Presently, the field employs scoring systems that incorporate multiple investigative results and findings, assigning a score accordingly. These systems include those developed by institutions such as The Musculoskeletal Infection Society (MSIS), Infectious Diseases Society of America (IDSA), International Consensus Meeting (ICM), and European Bone and Joint Infection Society (EBJIS) (Figures 2 to 4).
The application of the EBJIS criteria in infection detection may be particularly helpful. The EBJIS approach enables the identification of a broader spectrum of low-grade infections, thereby mitigating the issue of diagnostic ambiguity.118,119 Empirical evidence presented by Sigmund et al. lends credence to this assertion, indicating that the EBJIS methodology is associated with enhanced diagnostic precision.118 An examination of 206 patients earmarked for revision surgery, employing the criteria set forth by EBJIS, ICM and IDSA, was conducted to ascertain diagnostic accuracy.118
It was observed that the EBJIS criteria were instrumental in identifying 49% (n=101) of all PJIs, closely trailed by IDSA at 48% (n=99) and finally ICM at 42% (n=86). Beyond its superior PJI detection rate, the EBJIS criteria significantly diminished diagnostic ambiguity, as evidenced by a comparison of inconclusive diagnoses: 15% for ICM versus 8% for EBJIS. Notwithstanding concerns that EBJIS may result in misdiagnosing patients with aseptic failures as PJIs, Sigmund et al. stress the advantageous role it plays in enhancing surgical caution.118
The algorithmic approach proposed by Shohat et al. merits attention.120 Their study, analysing 422 cases using a diagnostic algorithm based on the MSIS criteria, demonstrated a sensitivity of 96.9% and specificity of 99.5% in diagnosing PJI, contrasting with a control group of 820 non-PJI patients.120 Interestingly, the alpha defensin test did not contribute to improved accuracy and was consequently excluded from routine testing in their algorithm. This study was grounded on the available methodologies at the time, thus sparking interest in the potential performance of a comparable algorithm using the EBJIS criteria.
Conclusion
The diagnostic landscape of Periprosthetic Joint Infections (PJIs) is riddled with complexities. An escalating concern in contemporary orthopaedics is the dwindling arsenal of antibiotics and concomitant increase in bacterial resistance, a reality which is only exacerbated by the growing rates of arthroplasties in an aging, increasingly frail populace. Consequently, a surge in PJIs is an unavoidable prospect in the near future. Therefore, it is not only imperative to bolster preventive measures, but also to strive towards refining the definition of infection, a cornerstone in advancing our practice.
Nonetheless, the trajectory for PJI diagnosis is imbued with optimism, as innovative avenues are persistently being explored. The advent of novel biomarkers presents promising potential in the sphere of PJI diagnosis. The infusion of artificial intelligence into orthopaedic diagnostics heralds an era of increased precision and improved outcomes. Adopting a holistic approach, by combining physical examination data, serum markers, synovial and intraoperative findings, we can significantly enhance our diagnostic accuracy. This synergistic approach heralds a brighter future for PJI diagnostics, an integral step towards improved patient outcomes and healthcare efficiency.
Summary points
-
PJI detection is a challenge due to varied presentations and the lack of a single definitive test, making a comprehensive, flexible approach to diagnosis necessary.
-
Multiple diagnostic tools are employed, each with limitations: Serum biomarkers and imaging techniques (X-ray, CT, MRI) offer variable accuracy, while synovial fluid analysis and nuclear medicine techniques show promise.
-
Advanced biomarkers and techniques like Alpha-Defensin, D-lactate, and sonication of biofilms offer increased diagnostic sensitivity, but challenges like cost, accessibility, and the risk of contamination persist.
-
Molecular diagnostic procedures like NGS offer improved detection of causative microorganisms and resistance genes, but are expensive and labour-intensive.
-
Scoring systems like EBJIS, MSIS, and IDSA are used to aid in diagnosis, with good support for EBJIS due to its ability to identify a broader range of infections and reduce diagnostic uncertainty.
Declaration of competing interests
The authors declare that they do not have any competing interests
Authors’ contributions
T Al-Jabri: Designed the article layout, drafted, edited and approved the manuscript.
M Ridha: Designed the article layout, produced the legends and drafted, edited and approved the manuscript.
MJ Wood: revised, edited and approved the manuscript.
B Kayani: edited and approved the manuscript.
C Jayadev: approved the manuscript.
RA McCulloch: helped design the layout, edited and approved the manuscript.
E Schemitsch: edited and approved the manuscript.
Funding
The authors did not receive any funding for this article
Ethical Approval
This was not required for this article
Acknowledgments
Not applicable
Consent for publication
Not applicable