Introduction
Total Knee Arthroplasty (TKA) is a well-established and successful surgical procedure for the treatment of injury or degenerative knee joint diseases.1
The procedure is designed to relieve joint pain, increase mobility, and improve quality of life.2 However, patients experience severe acute and chronic pain following TKA, which can detrimentally affect the quality and length of recovery.3 This can have indirect effects to post-operative rehabilitation and exercise which can eventually lead to reduced joint function. Regarding side effects, researchers have found that 15% of patients suffer from severe pain for up to 4 years post-operation.4 The evidence for postoperative pain is compounded by age-related loss of muscle mass and increased rates of obesity. The risk of post-operative disability increases as does the likelihood of more TKAs being undertaken.5,6 In total, 60% of TKA patients have been recorded to experience severe pain, whilst 30% reported moderate pain.7 Some patients even avoid the operation because of fear of acute postoperative pain.8
Parecoxib is a highly selective COX-2 inhibitor which can reduce the synthesis of peripheral prostaglandins to induce an analgesic effect and relieve inflammation without affecting platelet aggregation.9 Despite its many benefits, it has been associated with various side effects that must be considered. These side effects include ulcer and gastrointestinal bleeding, jaundice, abnormal liver function, high/low blood pressure, abnormal heart rhythm, swelling, rash, itching, back pain, disturbed sleeping, and heart failure.10
However, parecoxib has also been proven to be effective in the reduction of pain, following a plethora of operations that would normally cause significant pain, including TKA.11–13
In the current study, we aim to investigate the effect of parecoxib intravenous injections for TKA patients on vital indices. Our hypothesis is that the use of parecoxib is safe and is not accompanied with hemodynamic instability or any increase in blood transfusion requirements.
Materials and Methods
Study design
Patients undergoing elective TKA at the Asklepieion General Hospital of Voula, Athens, Greece were recruited. Exclusion criteria included: age < 40 or > 80 years old; ASA III or higher; obesity (>140 kg body weight); allergy to local anaesthetics; history of dependence on opioids; contraindications for subarachnoid anaesthesia or femoral block (coagulopathy, local infection, pre-existing neurological problems, patient refusal); contraindications to the administration of parecoxib, severe hepatic or renal disease (serum creatinine > 1.7 mg/dl).
All patients received continuous femoral nerve block under neurostimulation guidance. Then, spinal anaesthesia was performed to undertake the operation. Patients were randomly allocated, 1:1, into two groups in relation to the parecoxib/placebo administration. Group Parecoxib (Group P) had parecoxib 40 mg intravenously every 12 h. The first dose was administered 20 min prior to surgery completion and every 12 h within a 48 h period. Group Control (Group C) received the placebo drug (N/S 0.9 %) intravenously, instead of parecoxib. The rest of the analgesic medication was identical in both Groups. The randomization process was performed on the morning of surgery, when patients were randomized to one of the two groups using computer-generated tables. Hemodynamic measurements for each patient were performed by professional medical personnel. Systolic Arterial Pressure (SAP), Diastolic Arterial Pressure (DAP), Heart Rate (HR), Oxygen Saturation (Ox-Sat) and blood transfusion requirements were recorded. The hemodynamic data were measured at two different settings: every hour for up to 10 hours and at 15 min, 4, 8, 12, 24, 36 hours.
All drug solutions were prepared under aseptic conditions. All personnel involved in the clinical care (surgeons, anaesthesiologists, and nurses) and all the patients remained blinded to the substance and the treatment group assignment. Potential side effects of the drug were observed for and were dealt with immediately by trained medical personnel.
Continuous Femoral Nerve Block Under Neurostimulation Guidance
Local anaesthesia with lidocaine 1 % (0.5 mg/kg) was used to provide skin infiltration before the needle was inserted inferior to the inguinal crease, aiming at approximately 45° cephalad. Simultaneously, sedation with intravenous midazolam 0.05 mg/kg was administered. Continuous femoral nerve block was placed in a sterile fashion with neurostimulation guidance. In total, 20 ml of ropivacaine 0.75 % were injected as a bolus single shot. Then, a spinal subarachnoid block was placed in a standard, sterile technique. Later, in recovery, following TKA, a pump was connected to the catheter to facilitate an infusion of 0.2 % ropivacaine at 10 ml/hr.
Ethical considerations
Informed written consent was provided by all the patients prior to enrolment in the study. The study was also reviewed and approved by the Institutional Review Board and the Local Ethics Committee on human research and human studies at Aretaieion University Hospital with reference code S-138/15-06-10, and with the Helsinki Declaration. All data were analysed anonymously.
Statistical analysis
Continuous variables are presented as mean ± SD or medians and interquartile range depending on their distribution. Assessment of normality was performed though P-P, Q-Q diagrams and the Kolmogorov-Smirnov and Shapiro-Wilk tests. Categorical variables were summarized as absolute values and percentages. The independent samples t Test or the Mann-Whitney U test was used for between group comparisons of continuous data. For pairwise comparisons of proportions, the Chi-square or Fisher’s exact test were used for pairwise comparisons of proportions, as appropriate, along with their 95% CIs were calculated Data were analyzed with General Linear Models ANOVA and Bonferroni post-hoc test, which involves one factor between patients (factor “Group” with two levels) and one factor within patients (factor “Time” with appropriate levels, with repeated measures). In all hypotheses testing procedures the significance level was preset at p≤0.05.
Results
Ninety patients, in both Group P (n=45) and Group C (n=45), were followed for the predefined period and were included in the analysis. Seventy-five (83.3%) females were recruited, and sex distribution was similar between groups.
Descriptive data of HR, SAP, DAP are presented as mean and standard deviation in both Groups. Readings taken every hour, over a 10h period are provided in Table 1.
A slight decline in HR was recorded in both arms. Mean HR was 78 bpm at 1 hour and 70 bpm at 3 hours and the remaining time measured postoperatively. Using repeated measures ANOVA, the data did not show any significant statistical difference between the two cohorts (p value = 0.4, F = 0.715, df = 1).
The trend of SAP starts relatively high from the first hour at 150 mmHg and then decreased the third hour at 125 mmHg to remain at this point for the rest of the period. SAP trends were similar between Groups (p value = 0.437, F = 0.715, df = 1). Considering DAP fluctuations, a peak DAP at 15 minutes at 82 mmHg is noted that decreased to 70 mmHg at the fifth hour. Also, for DAP, the data presented no significant difference (p value = 0.535, F = 0.387, df = 1).
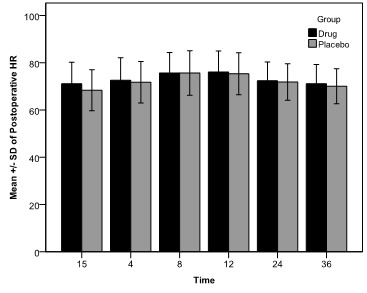
Extending the follow-up period at 36 hours, there was also no significant difference between the two groups (Table 2, p value = 0.568, F = 0.329, df = 1). The postoperative HR of patients from both study groups is presented over a period from 15 minutes until 36 hours post-surgery. Figure 1 illustrates a small relative bell curve, starting with the early measurements at 70 bpm, increasing and peaking between 8 – 12 hours at 75 bpm and then decreasing towards 70 bpm once again.
Regarding SAP, Figure 2 illustrates a trend that starts relatively low at 15 minutes with a mean 122 mmHg and increased until 8 hours to 140 mmHg which decreased to not clinically significant levels at 36 hours.
Analysis of the data showed no significant statistical difference between the two cohorts (p value = 0.286, F = 1.153, df = 1).
Figure 3 illustrates a starting DAP of 70 mmHg which slowly increased to 75 mmHg at 8 hours and remained at this value for the rest of the period. Recordings did not show any significant statistical difference between the two cohorts (p value = 0.547, F = 0.365, df = 1).
Postoperative oxygen saturation did not differ between Groups (p value = 0.350, F = 0.884, df = 1 and p value = 0.455, F = 0.563, df = 1 respectively).
The frequency of blood transfusion postoperatively was identical between groups. Thirteen (29%) patients and 14 (31.2%) required blood transfusion in the Parecoxib and Control Group respectively (p=0.6).
No significant side effects were noted. Four patients (n=4) experienced severe itching because of morphine, one drowsiness (n=1) and one dizziness (n=1). Otherwise, there were no obvious signs of allergic reactions to parecoxib or the local anaesthetic-ropivacaine.
Discussion
The purpose of this work was to evaluate the safety and any potential effect of the drug parecoxib on hemodynamic data and vital signs of participants undergoing TKA. Outcomes included HR, SAP, DAP, blood transfusion requirements as well as oxygen saturation.
Heart rate analysis
The perioperative HR of both drug and placebo participants showed no significant difference. The postoperative HR was also similar between the drug and placebo cohorts. Both cohorts were well within the range of average resting human heart rate of 60 – 100 bpm.
HR is a product of the autonomic nervous system and will be affected by signals sent to the brain. HR was recorded in efforts to determine pain quantification as researchers have already linked increased HR to pain.14 From the data, one could not suggest the intervention had an influence on the perceived pain experienced by the patients as the drug cohort did not record a statistically lower HR or HR variability compared to the placebo group. Of note, even though another study from the same research group demonstrated that the drug had a distinguishable pain relief effect, the HR rate of the drug cohort was not significantly lower than that of the placebo cohort.15
This could be attributed to the fact that the systems involved in regulating cardiovascular function are closely coupled with the perception of pain.16 This area of study is well documented and extensive research has already linked cardiovascular function to neural structures involved in pain sensation and autonomic control.3,17
Systolic and Diastolic Arterial Pressure
The perioperative SAP and DAP showed no statistically significant difference between the drug and placebo cohort. The data do, however, suggest that parecoxib did not affect hemodynamic stability. It may also be assumed that parecoxib does not provide any statistically significant analgesic effects to the participating cohort.
The variability of blood pressure and HR has been proven to be dependent on many other factors and combinations. This has been shown by researchers who analysed physiological and pathological states such as age, cardiovascular health, environment, and diet that influence blood pressure and HR. To ensure accurate results, any factors foresighted to interfere with the results, would have been screened for and the patient would be excluded from recruitment.18
As mentioned above, parecoxib has been known to cause side effects that concern hemodynamic indices, including high and low blood pressure, heart failure and abnormal heart rate.12 Contrary to the above, our study showed no difference since the hemodynamic data from both groups remained remarkably stable. This pattern has previously been observed in other studies including that of researchers who investigated the effects of pre-emptive analgesia with parecoxib sodium. They found that the drug could effectively subside levels of plasma stress hormones without conspicuous impact on haemodynamics.19
Oxygen saturation
Results concerning oxygen saturation revealed no statistically significant difference between the two cohorts. This outcome was expected, as oxygen saturation does not typically vary in states of pain compared to states of rest. This could be because the sympathetic response to pain may increase the respiratory rate, but the oxygen saturation of the blood will remain the same. This has been statistically verified by researchers who tested this hypothesis and observed no significant difference in a regression analysis of pain score and oxygen saturation levels.18
Transfusion requirements
Our study showed that transfusion requirements were similar between the two cohorts and generally low. A COX-2 inhibitor, such as parecoxib, has minimum effect on platelet function and does not cause excessive bleeding from the knee joint. No link has been identified between the administration of parecoxib and increased bleeding in a study that observed total blood loss following surgery after 48 hours.5,20
On the other hand, this is not in agreement with another study where there was a shift toward increased blood loss and transfusion needs at this predetermined period (5 patients in the parecoxib group versus 2 patients in the placebo group receiving red blood cell units). Moreover, patients who received parecoxib had a 1.5-fold higher rate of following surgery anaemia. This distinction, however, was not considered to be statistically important.21 Our study also showed that there is no significant difference between blood transfusion requirement between parecoxib and placebo treatment group of patients that have undergone TKA.
Despite the omission of tranexamic acid, transfusion requirements were low for both cohorts. This may have been due to the preset inclusion requirements that included good surgical technique, a high tolerance to haemoglobin infusion but ruled out low haemoglobin counts.
Limitations
One of the limitations of the study was that we followed very strict exclusion criteria. Patients with any sign of severe cardiovascular disease were excluded and we accepted only hypertension as comorbidity related with heart disease. Biochemical indices could also be studied, such as troponin but this would affect costs required to carry out the research.
Conclusions
In conclusion, the use of parecoxib use was safe and was not associated with any signs of hemodynamic disturbance or side effects. Vital signs remained stable and were not compromised at all during the whole perioperative period in both groups. Overall, the combination of CFNB and intravenous parecoxib is a safe and effective analgesia plan for patients undergoing TKA.
Acknowledgments
None.
Correspondence to
Despoina Sarridou, dodasarri@yahoo.gr, address: S. Kyriakidi 1, 54636, Thessaloniki, Greece
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript. DS, GC and AV have made substantial contributions to the conception or design of the work; DS, AG, HA, GC and AA were involved in the acquisition, analysis, or interpretation of data for the work; DS, AG, GC contributed to drafting the paper and HA, AA and AV contributed to reviewing it critically for important intellectual content. All authors have approved the final version to be published.
Conflict of interest
The authors report no conflict of interest.
Funding
No funding or sponsorship was received for this study or publication of this article.