Introduction
The Anterior Cruciate Ligament (ACL) serves as a crucial stabilizer within the knee joint, significantly contributing to maintaining joint stability during dynamic and high-impact activities.1 This ligament plays a vital role in preventing excessive forward movement and rotation of the tibia relative to the femur, which is essential for activities ranging from walking and running to jumping and sudden directional changes.2 Injuries to the ACL are not only common but can lead to severe consequences, including long-term disability, reduced quality of life, and an elevated risk of developing osteoarthritis in the affected joint.3 The impact of an ACL injury extends beyond the immediate physical damage, often necessitating extensive rehabilitation and, in many cases, surgical intervention to restore functionality and stability.4
Biophysically, the ACL is subjected to complex forces that include tension, shear, and torsion, all of which can contribute to its rupture under extreme conditions.5 These forces are particularly pronounced during activities that involve rapid acceleration, deceleration, or changes in direction, placing the ligament under significant strain.6 This review specifically addresses the biophysical dimensions of ACL injuries, providing a comprehensive examination of the biomechanical forces that stress the ligament, anatomical predispositions such as the shape and alignment of bones and ligaments that may increase susceptibility to injury, and physiological conditions including the health and strength of the ligament tissue.7
Anatomical factors play a critical role in the likelihood of ACL injuries.8 For example, variations in the intercondylar notch width, the angle of the femoral shaft, and the alignment of the tibial plateau can all influence the stress distribution on the ACL.9 Individuals with narrower notches or certain alignments might inherently face higher risks during physical activities.10 Additionally, the overall condition of the ligament tissue, influenced by factors such as age, previous injuries, and general health, can affect its ability to withstand mechanical stress.
Physiological conditions, including muscle strength, flexibility, and neuromuscular control, also significantly impact ACL integrity.11 Weak or imbalanced muscles, particularly in the quadriceps and hamstrings, can lead to improper knee mechanics, increasing the risk of ligament strain and injury. Furthermore, the neuromuscular response, or the body’s ability to react to sudden movements, is crucial in preventing ACL injuries.12 Improved neuromuscular control through targeted training can enhance joint stability and reduce injury risks.
By delving into these factors, the review aims to deepen the understanding of the complex interplay of elements that lead to ACL injuries, thereby informing the development of more targeted and effective strategies for the prevention and treatment of these debilitating injuries. This holistic approach not only addresses the immediate mechanical aspects of ACL injuries but also considers the broader anatomical and physiological context, leading to more comprehensive and effective intervention strategies.
Biophysical Stimulation for ACL Injury Treatement
Biophysical stimulation, when examined through the lens of molecular biophysics, reveals the intricate interplay of biological signals and physical forces that orchestrate tissue repair and regeneration at the cellular and molecular level.13 This field employs a diverse array of growth factors, stem cells, pharmacological agents, and physical modalities to specifically target and manipulate cellular pathways involved in tissue healing, influencing key molecular mechanisms such as gene expression, signaling transduction, and the regulation of the extracellular matrix.14
Growth factors such as RPR (Recombinant Platelet-Derived Growth Factor), BMP-2 (Bone Morphogenetic Protein-2), FGF (Fibroblast Growth Factor), VEGF (Vascular Endothelial Growth Factor), TGF-β (Transforming Growth Factor-beta), and BGF (Basic Growth Factor) play crucial roles in driving cellular processes by activating specific receptor-mediated signaling pathways that influence cellular proliferation, migration, differentiation, and survival. BMP-2, for instance, engages the SMAD signaling pathway by binding to serine/threonine kinase receptors on the cell surface, resulting in the phosphorylation and translocation of SMAD proteins into the nucleus.15 This leads to the transcription of genes involved in osteogenesis and bone formation.16 BMP-2 also interacts with the Wnt signaling pathway, promoting osteoblast differentiation through the stabilization of β-catenin, which acts as a transcriptional coactivator for osteogenic gene expression.
VEGF, through binding to VEGF receptors (VEGFR), activates PI3K/AKT and MAPK/ERK pathways, which are vital for endothelial cell survival, migration, and proliferation. These pathways converge to promote angiogenesis, a critical process in wound healing and tissue regeneration, ensuring that the regenerating tissue receives adequate blood supply for oxygen and nutrient delivery.17 PI3K/AKT signaling contributes to cellular survival by inhibiting pro-apoptotic factors such as BAD and by promoting cell cycle progression via the phosphorylation of mTOR. Meanwhile, MAPK/ERK signaling facilitates endothelial cell proliferation by phosphorylating downstream effectors such as c-Fos and Elk-1, which regulate the expression of pro-proliferative genes.18
TGF-β functions through its interaction with TGF-β receptors, which phosphorylate SMAD2/3, allowing these proteins to form complexes with SMAD4 that translocate to the nucleus and regulate gene expression. The TGF-β/SMAD pathway is critical in regulating fibroblast activity, promoting extracellular matrix deposition by upregulating the production of collagen, fibronectin, and proteoglycans. It also modulates immune responses by promoting the differentiation of regulatory T cells (Tregs), which suppress inflammation and promote tissue repair.19
Stem cell therapies, utilizing mesenchymal stem cells (MSCs), bone marrow-derived stem cells (BMSCs), and tissue-derived mesenchymal stem cells (TDMSCs), take advantage of the intrinsic ability of these cells to differentiate into various cell types and modulate their microenvironment through paracrine signaling. MSCs, for instance, can differentiate into osteoblasts, chondrocytes, and adipocytes through the activation of the Wnt/β-catenin pathway and the TGF-β/SMAD signaling axis.20 The Wnt/β-catenin pathway is essential for maintaining stem cell pluripotency and promoting osteogenic differentiation by stabilizing β-catenin, which then translocates into the nucleus to drive the expression of osteoblast-specific genes such as RUNX2 and SP7. The secretion of growth factors such as hepatocyte growth factor (HGF) and interleukin-10 (IL-10) by MSCs plays a critical role in modulating the inflammatory response, promoting angiogenesis, and recruiting endogenous progenitor cells to the site of injury.21
Pharmacological agents such as COX-2 inhibitors, GHK-Cu (Copper Peptide), PTH (Parathyroid Hormone), alendronate, and alpha2-macroglobulin target specific molecular pathways involved in tissue repair. COX-2 inhibitors reduce inflammation by blocking the cyclooxygenase-2 enzyme, which is involved in the synthesis of pro-inflammatory prostaglandins.22 This inhibition reduces the local inflammatory response, creating a more conducive environment for tissue regeneration by limiting the recruitment of inflammatory cells that could otherwise contribute to tissue damage. GHK-Cu, a naturally occurring tripeptide, has been shown to enhance the expression of genes associated with wound healing and skin regeneration by activating pathways involved in collagen synthesis, such as transforming growth factor-beta (TGF-β) and extracellular signal-regulated kinases (ERK).23
Biophysical modalities like Blood Flow Restriction (BFR), continuous passive motion, strength training, and intermittent negative pressure influence tissue healing at the molecular level by altering cellular ion channels, membrane potentials, and intracellular signaling.24 For example, BFR has been shown to stimulate muscle hypertrophy through the upregulation of the mammalian target of rapamycin (mTOR) pathway, which is critical for protein synthesis and muscle regeneration.25 Electromagnetic fields, including low-intensity laser therapy and pulsed electromagnetic field therapy (PEMF), enhance osteogenesis by upregulating the expression of osteogenic genes and proteins through the activation of the Wnt/β-catenin pathway, facilitating the differentiation of mesenchymal progenitor cells into osteoblasts.26
Electrical stimulation, such as TENS (Transcutaneous Electrical Nerve Stimulation) and NMES (Neuromuscular Electrical Stimulation), modulates neuronal and muscular activity by affecting the excitability of neurons and muscle fibers.27 These modalities influence ion channel activity, increasing intracellular calcium levels, which are crucial for activating downstream signaling pathways such as calcineurin/NFAT, leading to increased protein synthesis, growth factor production, and accelerated tissue repair.28 Electrical stimulation has also been shown to enhance mitochondrial biogenesis by activating peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), a key regulator of energy metabolism and mitochondrial function in regenerating tissues.29
Mechanical stimulation techniques, such as pressotherapy, whirlpool therapy, and shockwave therapy, promote tissue regeneration by improving blood flow, reducing inflammation, and inducing controlled microtrauma that stimulates cellular repair mechanisms.30 Mechanical forces exerted on cells activate mechanotransduction pathways, whereby mechanical signals are converted into biochemical responses. This process involves the activation of integrins and other mechanosensitive proteins, which transmit mechanical stimuli to the cytoskeleton, triggering signaling cascades such as the MAPK/ERK pathway.31 This cascade is crucial for cell proliferation, differentiation, and the synthesis of extracellular matrix proteins, essential for tissue repair and regeneration.32
In conclusion, the molecular integration of biophysics into biophysical stimulation therapies offers a comprehensive approach to enhancing tissue repair and regeneration. By targeting key molecular pathways such as Wnt/β-catenin, SMAD, PI3K/AKT, and MAPK/ERK, these therapies can modulate gene expression, cell signaling, and protein synthesis to optimize healing outcomes. This multidisciplinary approach, which addresses both the biological and physical dimensions of tissue healing, ensures that each aspect of the regenerative process—from cellular signaling and extracellular matrix remodeling to the mechanical environment—is holistically managed, providing an advanced strategy for medical treatment and rehabilitation.
Biomechanical Factors
Biomechanical factors play a pivotal role in the occurrence and severity of Anterior Cruciate Ligament (ACL) injuries. Understanding these factors involves examining the various forces and moments acting on the knee joint during physical activities, which can lead to ACL strain or rupturę.33 The primary biomechanical forces that affect the ACL include axial loading, anterior tibial translation, and rotational forces. Axial loading refers to the vertical forces exerted on the knee when the body is in motion, such as during landing from a jump or sudden deceleration. Anterior tibial translation involves the forward movement of the tibia relative to the femur, which places significant stress on the ACL, especially during activities like cutting, pivoting, or sudden stops. Rotational forces, particularly internal and external rotation of the tibia, further compound the stress on the ACL, making it more susceptible to injury during dynamic movements.34
Kinematic and kinetic analyses provide detailed insights into how these forces interact with the anatomical structures of the knee. Kinematics focuses on the motion of the knee joint, analyzing the angles and velocities of limb movements without considering the forces that cause tchem.35 This analysis helps identify specific movement patterns that increase the risk of ACL injury, such as excessive knee valgus (inward collapse of the knee) or improper landing mechanics. Kinetics, on the other hand, examines the forces and torques acting on the knee, providing a deeper understanding of how different movements generate stress on the ACL.36 Studies have shown that certain athletic maneuvers, such as rapid changes in direction, pivoting on a planted foot, or landing from a jump with an extended knee, significantly elevate the risk of ACL injury due to the high biomechanical demands they place on the ligament.37
Moreover, muscle strength and coordination are critical biomechanical factors influencing ACL injury risk. The quadriceps and hamstrings play essential roles in stabilizing the knee joint and controlling its movement.38 Imbalances in the strength or timing of these muscles can lead to improper joint mechanics and increased ACL strain. For instance, overdominance of the quadriceps relative to the hamstrings during high-impact activities can cause excessive anterior tibial translation, putting the ACL at greater risk. Neuromuscular control, which involves the coordination of muscle activation patterns to stabilize the joint, is equally important. Deficits in neuromuscular control, often observed in fatigued or untrained athletes, can result in compromised knee stability and a higher likelihood of injury.39
Additionally, external factors such as footwear, playing surface, and environmental conditions can influence the biomechanical environment of the knee.40 For example, playing on artificial turf has been associated with a higher incidence of ACL injuries compared to natural grass, likely due to the increased traction and reduced ability to slide, leading to greater rotational forces on the knee. Similarly, improper footwear that fails to provide adequate support or traction can alter movement mechanics and increase the risk of injury.41
Understanding the interplay of these biomechanical factors is crucial for developing effective prevention strategies. Training programs that focus on improving muscle strength, particularly the balance between quadriceps and hamstrings, enhancing neuromuscular control, and promoting proper movement techniques can significantly reduce the risk of ACL injuries.42 Furthermore, considerations for appropriate footwear and playing surfaces can also play a role in mitigating biomechanical risks. By addressing these factors, athletes can reduce their vulnerability to ACL injuries and improve their overall knee joint health.43
In addition to biomechanical factors, the molecular biophysics of the ACL plays a crucial role in understanding injury mechanisms and developing effective prevention and treatment strategies.44 The ACL is composed primarily of collagen fibers, which provide tensile strength and flexibility. These fibers are organized in a hierarchical structure, from the molecular level to the macroscopic level, contributing to the ligament’s mechanical properties.45
At the molecular level, collagen molecules form triple helices, which then assemble into fibrils. These fibrils bundle together to create fibers, and fibers aggregate to form the ligament.46 The cross-linking between collagen molecules, facilitated by enzymatic processes, provides additional strength and stability to the ligament. Any disruption in this hierarchical structure, such as through mechanical overload or biochemical degradation, can compromise the integrity of the ACL and increase the risk of injury.47
Molecular biophysics also involves understanding the role of proteoglycans and other extracellular matrix components in maintaining the structural integrity and function of the ACL.48 Proteoglycans, such as decorin and biglycan, interact with collagen fibrils to regulate fibrillogenesis and maintain tissue hydration and viscoelastic properties. Changes in the composition or organization of these matrix components can affect the mechanical behavior of the ACL and its susceptibility to injury.49
Furthermore, molecular signaling pathways play a critical role in the response of ACL tissue to mechanical stress and injury.50 Cellular mechanotransduction mechanisms, which involve the conversion of mechanical signals into biochemical responses, are essential for maintaining ligament homeostasis and initiating repair processes following injury.51 For example, integrins, which are transmembrane receptors, mediate the interaction between the extracellular matrix and the cytoskeleton, influencing cell behavior and tissue remodeling. Understanding these molecular pathways can provide insights into the development of targeted therapies to enhance ligament healing and regeneration.52
Overall, a comprehensive understanding of both the biomechanical and molecular biophysical factors influencing ACL injuries is essential for developing effective prevention and treatment strategies. Integrating knowledge from these domains can lead to the development of more targeted interventions, such as biomechanical training programs, molecular therapies to enhance tissue repair, and novel biomaterials for ligament reconstruction. By addressing the complex interplay of forces and molecular processes that contribute to ACL injuries, it is possible to improve outcomes for individuals at risk of or recovering from these debilitating injuries (Table 2).
1. Kinematic and Kinetic Analysis
Kinematic and kinetic analyses consistently demonstrate that excessive anterior tibial translation and internal tibial rotation significantly contribute to anterior cruciate ligament (ACL) strain. These biomechanical movements are critical in understanding the mechanisms that lead to ACL injuries. Anterior tibial translation refers to the forward movement of the tibia in relation to the femur, while internal tibial rotation involves the inward twisting of the tibia. Both movements place considerable stress on the ACL, making it more susceptible to injury.53
Non-contact mechanisms are particularly noteworthy in the context of ACL injuries. These mechanisms include sudden deceleration, pivoting, and landing from a jump. Sudden deceleration occurs when an athlete abruptly reduces speed, which often happens during activities such as running or changing directions quickly.54 This rapid change in momentum can cause excessive strain on the ACL as the tibia moves forward against the femur.55
Pivoting, another common non-contact mechanism, involves rotating or twisting the body while the foot remains planted. This action can cause internal tibial rotation, leading to increased tension on the ACL.56 Similarly, landing from a jump, especially with improper technique or insufficient muscle strength, can result in significant anterior tibial translation and internal rotation, both of which are detrimental to the integrity of the ACL.57
High-speed video analysis and motion capture studies have been instrumental in identifying these movements as critical risk factors for ACL injuries. These technologies allow researchers to observe and measure the precise kinematic and kinetic variables involved in sports movements.58 By analyzing the motion patterns of athletes during activities that commonly lead to ACL injuries, researchers can pinpoint the exact moments and movements that place the ACL at risk. This detailed understanding is crucial for developing preventive strategies and improving training techniques to reduce the incidence of ACL injuries in athletes.59
From a biophysical perspective, understanding the material properties and structural composition of the ACL is essential. The ACL is a complex ligament composed primarily of collagen fibers, which provide tensile strength and elasticity. These properties allow the ACL to withstand the forces exerted during physical activities. However, when the forces exceed the ligament’s capacity, microtears or complete ruptures can occur.60
Biomechanical modeling and simulations further enhance our understanding of ACL strain during various activities. These models consider factors such as muscle forces, joint angles, and external loads to predict the stress distribution within the ACL. By integrating kinematic and kinetic data with biomechanical models, researchers can simulate different scenarios and assess the effectiveness of various preventive measures, such as bracing or specific training regimens.61
Additionally, the role of neuromuscular control in ACL injury prevention cannot be overlooked. Proper activation and coordination of the muscles surrounding the knee joint are crucial for stabilizing the tibia and reducing undue stress on the ACL. Training programs that focus on improving neuromuscular control, such as plyometrics, balance exercises, and proprioceptive training, have been shown to decrease the risk of ACL injuries.62
In biophysics, the application of principles such as stress-strain relationships and viscoelasticity is crucial for understanding ACL mechanics. The stress-strain relationship describes how the ACL deforms under various loads, providing insight into its mechanical properties such as stiffness and resilience. Viscoelasticity, a property of biological tissues, refers to the time-dependent response of the ACL to stress. This means that the ligament’s response to loading is not only dependent on the magnitude of the load but also on the rate at which the load is applied and the duration of the load.63
Advanced imaging techniques, such as magnetic resonance imaging (MRI) and ultrasound elastography, offer detailed views of the ACL’s internal structure and its response to mechanical forces. MRI can reveal microstructural changes and tears, while ultrasound elastography measures tissue stiffness, providing real-time feedback on the ligament’s conditio.64
Furthermore, the application of computational fluid dynamics (CFD) in biophysics can shed light on the fluid environment within the knee joint. Synovial fluid dynamics influence the lubrication and nutrition of the ACL, affecting its health and response to mechanical stress. Understanding the interaction between fluid dynamics and ligament mechanics can lead to better injury prevention and treatment strategies.65
In summary, the integration of kinematic and kinetic analyses, high-speed video analysis, motion capture technology, biomechanical modeling, advanced imaging techniques, and computational fluid dynamics offers a comprehensive biophysical approach to understanding and preventing ACL injuries. This multifaceted strategy not only helps identify the underlying mechanisms of ACL strain but also guides the development of targeted interventions to enhance athlete safety and performance.
2. Impact of External Loads
External forces, including valgus stress and axial loading, significantly increase ACL loading, particularly during dynamic activities like cutting maneuvers and sidestepping. Valgus stress refers to the force that causes the knee to bend inward, creating a knock-knee alignment, while axial loading involves forces applied along the length of the leg. Both types of stress can dramatically enhance the strain on the ACL, increasing the risk of injury.66
Valgus stress is commonly encountered during lateral movements and sudden directional changes. When an athlete performs a cutting maneuver, the rapid lateral shift in body weight can push the knee into a valgus position. This inward angling of the knee, coupled with the rotational forces generated by the movement, places immense pressure on the ACL. The ligament is forced to resist not only the forward translation of the tibia but also the inward collapse and rotation of the knee joint.67
Axial loading, on the other hand, occurs during activities that involve vertical forces, such as landing from a jump or when an athlete’s foot strikes the ground with substantial force. This vertical compression can exacerbate the anterior tibial translation, further stressing the ACL. When combined with improper landing techniques or muscle imbalances, axial loading can lead to catastrophic failure of the ligament.68
Finite element modeling has been pivotal in illustrating how these external loads affect the ACL. These computational models simulate the complex interactions between bones, ligaments, and muscles under various loading conditions. By creating a virtual environment that replicates the physical properties and movements of the knee, researchers can visualize the stress distribution across the ACL. These simulations reveal that valgus stress and axial loading significantly elevate the tension within the ligament, highlighting the critical points where failure is most likely to occur.69
Cadaveric studies complement these models by providing empirical data on how the ACL responds to external loads. In controlled laboratory settings, cadaver knees are subjected to forces that mimic real-life dynamic activities. These experiments have shown that both valgus stress and axial loading can lead to partial or complete ACL tears, validating the findings from finite element models. By analyzing the failure patterns in cadaveric specimens, researchers can better understand the thresholds at which the ACL can no longer withstand the applied forces.70
The integration of finite element modeling and cadaveric studies offers a comprehensive view of the biomechanical impact of external loads on the ACL. This combination of theoretical and practical approaches enables a deeper understanding of the injury mechanisms and helps in developing more effective prevention and rehabilitation strategies. For instance, insights from these studies can inform the design of training programs that enhance knee stability and strength, thereby reducing the risk of valgus and axial loading during athletic activities.71
From a biophysical standpoint, understanding the material properties and structural behavior of the ACL under these external loads is crucial. The ACL exhibits viscoelastic properties, meaning its response to stress is time-dependent and involves both elastic and viscous components.72 When subjected to rapid loading, such as during cutting maneuvers or landing from a jump, the ligament’s elastic response is predominant, attempting to return to its original shape. However, prolonged or repetitive loading can cause viscoelastic creep, where the ligament slowly deforms over time, leading to microtears and eventual failure.73
The collagen fibers in the ACL are organized in a crimped pattern, which allows them to stretch and absorb forces. Under excessive loading, the crimp pattern straightens, and the fibers align in the direction of the force. This structural adaptation helps the ligament manage high stress, but beyond a certain threshold, the collagen fibers can rupture, leading to ligament failure.74
Advanced imaging techniques, such as magnetic resonance imaging (MRI) and ultrasound elastography, provide detailed insights into the internal structure and mechanical properties of the ACL. MRI can reveal changes in the ligament’s microstructure, such as fiber alignment and density, which are indicative of stress and damage. Ultrasound elastography measures the stiffness of the ACL, offering real-time feedback on its viscoelastic properties and the impact of external loads.75
Biomechanical modeling also extends to the cellular level, where the response of ACL fibroblasts (the cells responsible for maintaining ligament integrity) to mechanical stress is studied. Fibroblasts play a crucial role in the synthesis and repair of collagen fibers. Understanding how these cells respond to different loading conditions can inform strategies to enhance ligament healing and resilience.76
Furthermore, the fluid dynamics within the knee joint, particularly the role of synovial fluid in lubricating and nourishing the ACL, are critical biophysical factors. Synovial fluid reduces friction and distributes forces within the joint, helping to protect the ACL from excessive stress. Computational fluid dynamics (CFD) models can simulate the behavior of synovial fluid under different loading scenarios, providing insights into how fluid flow influences ligament health and injury risk.77
Molecular biophysics adds another layer of understanding by examining the molecular structure and behavior of the ACL’s components. The primary structural protein in the ACL is collagen, which forms a triple-helix structure that provides strength and flexibility.78 Molecular dynamics simulations can model the behavior of collagen molecules under stress, revealing how molecular bonds within the collagen fibers stretch, break, and reform in response to mechanical loads. These simulations can identify weak points at the molecular level where failure is likely to initiate, providing targets for therapeutic intervention.79
Additionally, molecular biophysics can elucidate the role of other extracellular matrix (ECM) proteins, such as elastin and proteoglycans, which contribute to the ligament’s elasticity and resilience.80 Elastin fibers allow the ligament to stretch and recoil, while proteoglycans help maintain tissue hydration and resistance to compressive forces. Understanding how these molecules interact and respond to mechanical stress can inform the development of biomaterials for ACL repair and reconstruction.81
Furthermore, the signaling pathways that regulate the cellular response to mechanical stress are critical for maintaining ACL integrity. Mechanotransduction, the process by which cells convert mechanical stimuli into biochemical signals, involves various proteins and ion channels that respond to changes in mechanical load.82 Research into these pathways can reveal how mechanical stress affects gene expression, protein synthesis, and cellular behavior, providing insights into how to promote healing and prevent injury at the molecular level.83
In summary, the impact of external loads, including valgus stress and axial loading, on ACL strain is significant. The integration of finite element modeling, cadaveric studies, advanced imaging techniques, and biophysical analyses, including molecular biophysics, offers a comprehensive understanding of how these forces contribute to ligament failure. This multifaceted approach is essential for developing targeted prevention and rehabilitation strategies, enhancing athlete safety, and improving outcomes in ACL injury management. By combining insights from macro-scale biomechanics and molecular-level studies, researchers can develop more effective interventions to protect and repair the ACL.
3. Muscle Activation Patterns
Altered muscle activation patterns, particularly in the quadriceps and hamstrings, have been implicated in increasing ACL load. Electromyography (EMG) studies show that imbalanced or delayed muscle activation can result in greater strain on the ACL during dynamic movements. The quadriceps and hamstrings play a crucial role in stabilizing the knee joint, and any disruption in their coordinated function can significantly impact ACL loading.84
The quadriceps, located at the front of the thigh, are primarily responsible for knee extension. When activated, they generate a forward force on the tibia, which, if excessive or not counterbalanced by the hamstrings, can lead to increased anterior tibial translation. This anterior shift of the tibia places additional tension on the ACL, heightening the risk of injury.85 EMG studies have shown that during activities such as landing from a jump or performing a cutting maneuver, an overactive quadriceps can contribute to this forward translation, especially if the hamstrings do not adequately co-contract to stabilize the joint.86
The hamstrings, located at the back of the thigh, counteract the quadriceps by providing knee flexion and resisting anterior tibial translation. Proper activation and timing of the hamstrings are essential for maintaining knee stability.87 If the hamstrings activate too late or with insufficient force, they fail to adequately oppose the forward pull of the quadriceps, resulting in greater ACL strain. EMG data has demonstrated that individuals with delayed or reduced hamstring activation are more prone to ACL injuries, particularly during high-risk activities such as sudden deceleration, pivoting, or changing directions quickly.88
In addition to the quadriceps and hamstrings, other muscle groups, such as the gastrocnemius (calf muscles) and gluteal muscles, also contribute to knee stability. The gastrocnemius assists in controlling knee flexion and extension, while the gluteal muscles help stabilize the pelvis and lower limb. Dysfunction or weakness in these muscles can further exacerbate imbalanced muscle activation patterns, indirectly increasing the load on the ACL.89
Neuromuscular control, which involves the coordinated activation of muscles in response to sensory input, is a key factor in preventing ACL injuries. Training programs that enhance neuromuscular control aim to improve the timing, strength, and coordination of muscle activation patterns. Exercises such as plyometrics, agility drills, and balance training can help athletes develop better muscle control, reducing the risk of imbalanced activation and subsequent ACL strain.90
Advanced EMG techniques, including surface EMG and intramuscular EMG, provide detailed insights into muscle activation patterns. Surface EMG involves placing electrodes on the skin overlying the muscles, while intramuscular EMG uses fine-wire electrodes inserted directly into the muscle tissue. These techniques allow researchers to measure muscle activity with high precision, identifying specific activation patterns that contribute to ACL loading.91
Furthermore, integrating EMG data with motion capture technology and biomechanical modeling can enhance our understanding of muscle activation’s impact on ACL strain. By analyzing the synchronized movements and muscle activations during dynamic activities, researchers can develop comprehensive models that predict how different activation patterns influence ACL loading. These models can inform the design of targeted training programs and interventions to optimize muscle function and protect the ACL.92
Molecular biophysics adds another dimension by examining how muscle fibers and their contractile proteins, such as actin and myosin, respond to neural signals and mechanical loads. Understanding the molecular mechanisms of muscle contraction and the role of motor units (groups of muscle fibers controlled by a single motor neuron) can provide insights into how muscle activation patterns affect joint stability. For example, variations in motor unit recruitment and firing rates can influence the force generated by muscles, affecting their ability to stabilize the knee and protect the ACL.93
At the molecular level, muscle contraction begins with the sliding filament theory, where myosin heads bind to actin filaments and pull them toward the center of the sarcomere, the basic unit of a muscle’s striated muscle fiber. This process is powered by adenosine triphosphate (ATP) hydrolysis. The efficiency and force of muscle contraction depend on the proper functioning and interaction of these molecular components. Disruptions or inefficiencies in this process can result in weaker muscle contractions and poor joint stabilization, contributing to increased ACL strain.94
The role of calcium ions (Ca2+) in muscle contraction is also critical. When a muscle is stimulated by a nerve impulse, Ca2+ is released from the sarcoplasmic reticulum into the cytoplasm of the muscle fiber.95 This increase in Ca2+ concentration triggers the interaction between actin and myosin, leading to muscle contraction. Any abnormalities in calcium handling, such as delayed release or reuptake, can impair muscle function and coordination, thereby affecting knee stability and increasing the risk of ACL injury.96
Research into the molecular signaling pathways that regulate muscle adaptation and hypertrophy (growth) can also inform strategies to enhance muscle strength and coordination. For instance, pathways involving mechanotransduction, where mechanical signals are converted into biochemical responses, play a significant role in muscle adaptation.97 Proteins such as integrins and focal adhesion kinase (FAK) are involved in these pathways, linking the extracellular matrix to the intracellular cytoskeleton and transmitting mechanical signals that promote muscle growth and adaptation.98
Molecular studies also explore the role of satellite cells in muscle repair and growth. Satellite cells are a type of stem cell found in muscle tissue that become activated in response to muscle damage or stress. Once activated, they proliferate and differentiate into myoblasts, which then fuse to form new muscle fibers or repair damaged ones. Understanding the molecular cues that regulate satellite cell activity can inform strategies to enhance muscle recovery and growth, potentially reducing the risk of muscle imbalances that contribute to ACL injuries.99
Additionally, molecular biophysics research examines the effects of various biochemical factors, such as hormones and growth factors, on muscle function and adaptation. For example, insulin-like growth factor 1 (IGF-1) plays a crucial role in muscle growth and repair by promoting protein synthesis and inhibiting protein degradation. Studies on how IGF-1 and other growth factors influence muscle adaptation can lead to new approaches for enhancing muscle function and preventing injuries.100
In summary, altered muscle activation patterns, particularly in the quadriceps and hamstrings, play a critical role in increasing ACL load. EMG studies have highlighted the importance of balanced and timely muscle activation in protecting the ACL during dynamic movements. By integrating advanced EMG techniques, motion capture technology, biomechanical modeling, and molecular biophysics, researchers can develop comprehensive strategies to optimize muscle function and reduce the risk of ACL injuries. These approaches are essential for designing effective prevention and rehabilitation programs that enhance neuromuscular control and ensure the stability and integrity of the knee joint. Molecular biophysics, in particular, provides valuable insights into the underlying mechanisms of muscle function and adaptation, paving the way for innovative interventions to enhance athletic performance and protect against injuries.
Anatomical Factors
Anatomical factors play a significant role in the risk of ACL injuries. Variations in the anatomy of the knee and lower extremities can influence the biomechanics and loading patterns, predisposing certain individuals to higher ACL strain and potential injury. These factors include the geometry of the knee joint, the alignment of the lower limb, and the structural characteristics of the ACL itself.101
One critical anatomical factor is the intercondylar notch width, the groove at the distal end of the femur through which the ACL passes. A narrower intercondylar notch can restrict the space available for the ACL, increasing the likelihood of impingement and subsequent injury during dynamic movements. Studies have shown that individuals with a narrower notch width are at a higher risk of ACL tears, likely due to the increased mechanical constraints placed on the ligament.102
The tibial slope, or the angle of the tibial plateau, is another crucial anatomical factor. A steeper posterior tibial slope has been associated with increased anterior tibial translation during weight-bearing activities, which places additional strain on the ACL. This increased slope can lead to a higher incidence of ACL injuries, especially in activities involving sudden deceleration or changes in direction.103
Lower limb alignment, particularly the Q-angle (quadriceps angle), is also influential. The Q-angle is the angle formed by a line drawn from the anterior superior iliac spine to the center of the patella and another line from the center of the patella to the tibial tubercle. A larger Q-angle can predispose individuals, especially females, to ACL injuries by increasing lateral forces on the knee, leading to greater valgus stress and internal tibial rotation. This misalignment creates a biomechanical environment that is more susceptible to ACL strain during dynamic activities.104
The size and shape of the ACL itself are anatomical factors that affect its susceptibility to injury. Variations in the cross-sectional area and the length of the ligament can influence its mechanical properties and its ability to withstand forces. A smaller or thinner ACL may be less capable of handling the high loads encountered during athletic activities, making it more prone to tears.105
Gender differences in anatomy also contribute to varying ACL injury risks. Females typically have a wider pelvis, greater Q-angle, and more significant ligamentous laxity compared to males. These differences result in altered biomechanics and increased valgus alignment, which can elevate the risk of ACL injuries. Additionally, hormonal variations, particularly fluctuations in estrogen levels, have been shown to affect ligament laxity and strength, further contributing to the higher incidence of ACL injuries in females.106
Advanced imaging techniques, such as magnetic resonance imaging (MRI) and computed tomography (CT), have been instrumental in studying these anatomical factors in detail. MRI provides high-resolution images of soft tissues, allowing for precise measurements of the ACL, intercondylar notch, and tibial slope. CT scans offer detailed views of the bone structures, facilitating accurate assessments of lower limb alignment and notch geometry. These imaging modalities are crucial for identifying anatomical variations that may predispose individuals to ACL injuries.107
Molecular biophysics offers further insights into how these anatomical factors influence ACL mechanics at a microscopic level. The extracellular matrix (ECM) of the ACL, composed primarily of collagen fibers, provides structural support and strength. Variations in the composition and organization of the ECM can affect the ligament’s mechanical properties. For instance, differences in collagen cross-linking and fiber orientation can influence the ACL’s tensile strength and elasticity, impacting its ability to withstand mechanical loads.108
Moreover, molecular studies on the genetic factors that regulate the development and maintenance of knee joint structures can provide valuable information. Genetic variations can influence the expression of proteins involved in collagen synthesis, ECM organization, and bone morphology. Understanding these genetic factors can help identify individuals at higher risk of ACL injuries and inform personalized prevention strategies.109
Research into the mechanotransduction pathways that govern how cells within the ACL respond to mechanical stress is also critical. Mechanotransduction involves the conversion of mechanical signals into biochemical responses, leading to cellular adaptations that strengthen the ligament. Identifying key molecules and pathways involved in this process can inform strategies to enhance ACL resilience and repair.110
In summary, anatomical factors significantly impact ACL injury risk by influencing knee biomechanics and loading patterns. Variations in intercondylar notch width, tibial slope, lower limb alignment, and the structural characteristics of the ACL itself can predispose individuals to higher strain and potential injury. Advanced imaging techniques and molecular biophysics provide valuable insights into these factors, facilitating the development of targeted prevention and treatment strategies to reduce the incidence of ACL injuries. Understanding the interplay between anatomy, biomechanics, and molecular mechanisms is essential for improving athlete safety and performance (Table 3).
1. Femoral Notch Width
A narrower femoral notch has been associated with a higher risk of ACL injury. The femoral notch, or intercondylar notch, is the groove at the distal end of the femur through which the ACL passes. This anatomical structure plays a critical role in providing space for the ACL to function properly. When the femoral notch is narrower, the available space for the ACL is reduced, which can lead to several biomechanical challenges that increase the risk of injury.111
MRI and cadaveric studies have provided substantial evidence supporting the correlation between a narrower femoral notch and a higher incidence of ACL injuries. MRI allows for detailed visualization of the knee’s internal structures, enabling precise measurements of the notch width. These studies have consistently shown that individuals with a narrower notch have a higher likelihood of experiencing ACL impingement, particularly during high-stress activities such as cutting, pivoting, and sudden deceleration. Impingement refers to the mechanical pinching or compression of the ACL within the femoral notch, which can cause significant stress and strain on the ligament.112
During dynamic movements, the knee joint undergoes complex motions that involve anterior tibial translation and rotational forces. In a knee with a narrow femoral notch, these movements can cause the ACL to repeatedly rub against the bony edges of the notch. Over time, this impingement can weaken the ACL fibers, making them more susceptible to microtears and eventual rupture. Cadaveric studies have demonstrated that when the ACL is subjected to repetitive impingement in a narrow notch, it exhibits signs of wear and damage, providing a direct link between notch width and ACL vulnerability.113
The biomechanical implications of a narrow femoral notch extend beyond impingement. The limited space can also restrict the ACL’s ability to move freely during knee flexion and extension. This restriction can alter the ligament’s natural mechanics, leading to abnormal stress distribution within the ACL fibers. Such stress concentrations can exacerbate the risk of injury, especially during activities that involve rapid changes in direction or high-impact landings.114
From a developmental perspective, the width of the femoral notch is influenced by both genetic and environmental factors. Genetic predispositions can result in variations in notch morphology, with some individuals naturally having narrower notches. Environmental factors, such as physical activity levels during growth periods, can also affect bone development and the eventual shape of the femoral notch. Understanding these developmental influences can help identify individuals who may be at a higher risk for ACL injuries based on their anatomical characteristics.115
Advanced imaging techniques like MRI provide crucial insights into the structural and functional aspects of the femoral notch. By analyzing the notch width in conjunction with other anatomical factors, clinicians and researchers can develop more accurate risk assessments for ACL injuries. For example, combining notch width measurements with evaluations of tibial slope and lower limb alignment can create a comprehensive profile of an individual’s knee biomechanics, allowing for tailored prevention strategies.116
Interventions aimed at reducing the risk of ACL injuries in individuals with narrow femoral notches may include targeted strength and conditioning programs. These programs can focus on enhancing the stability of the knee joint by strengthening the surrounding musculature, such as the quadriceps, hamstrings, and gluteal muscles. Improved muscle strength and coordination can help mitigate the effects of a narrow notch by providing better support and reducing the likelihood of impingement.117
Additionally, biomechanical training that emphasizes proper movement patterns and landing techniques can be beneficial. Educating athletes on how to safely decelerate, pivot, and land can minimize the stress placed on the ACL and reduce the risk of injury. For example, teaching athletes to engage their hamstrings and gluteal muscles during dynamic movements can help counteract the forces that lead to anterior tibial translation and ACL strain.118
Surgical techniques, such as notchplasty, have also been explored as potential interventions for individuals with narrow femoral notches. Notchplasty involves surgically widening the femoral notch to provide more space for the ACL, thereby reducing the risk of impingement. This procedure is typically considered for individuals who have experienced recurrent ACL injuries and have been identified as having a narrow notch.119
Molecular biophysics provides deeper insights into the implications of a narrow femoral notch on ACL health. At the molecular level, the stress and strain on the ACL fibers due to impingement can lead to microstructural changes within the collagen matrix. The ACL is primarily composed of type I collagen, a protein that provides tensile strength and structural integrity. Repeated impingement and mechanical stress can cause collagen fibrils to undergo deformation, affecting their alignment and cross-linking patterns. This degradation at the molecular level weakens the overall structure of the ACL, making it more susceptible to tears.120
Moreover, molecular studies have shown that the response of ACL cells, known as fibroblasts, to mechanical stress involves complex signaling pathways. Mechanical loading triggers mechanotransduction processes, where mechanical stimuli are converted into biochemical signals within the cells. This process involves integrins, which are transmembrane receptors that connect the extracellular matrix (ECM) to the cytoskeleton. When integrins detect mechanical strain, they activate intracellular signaling cascades that can lead to changes in gene expression and protein synthesis.121
One crucial signaling pathway activated by mechanical stress is the MAPK/ERK pathway. This pathway is involved in the cellular response to a variety of stressors, including mechanical load, and plays a role in regulating cell proliferation, differentiation, and apoptosis. In the context of ACL impingement, chronic activation of stress-responsive pathways can lead to an imbalance between ECM synthesis and degradation, further compromising the ligament’s integrity.122
Research into the role of matrix metalloproteinases (MMPs) in ACL degeneration provides additional molecular insights. MMPs are enzymes that break down collagen and other ECM components. Under normal conditions, MMP activity is tightly regulated to maintain tissue homeostasis. However, excessive mechanical stress and impingement can upregulate MMP expression, leading to increased collagen degradation and weakening of the ACL. Understanding the regulation of MMPs in response to mechanical stress could inform therapeutic strategies to mitigate ACL damage.123
Another area of interest in molecular biophysics is the role of oxidative stress in ACL injuries. Mechanical stress and impingement can generate reactive oxygen species (ROS), which are chemically reactive molecules containing oxygen. ROS can cause oxidative damage to cellular components, including lipids, proteins, and DNA. In the ACL, oxidative stress can impair fibroblast function and promote ECM degradation. Antioxidant therapies aimed at reducing oxidative stress could potentially protect the ACL from damage associated with a narrow femoral notch.124
Furthermore, the role of mechanobiology in ACL health is an essential aspect of molecular biophysics. Mechanobiology explores how mechanical forces influence cellular behavior and tissue remodeling. In the case of the ACL, mechanobiology studies can reveal how mechanical loading patterns affect cellular activities such as proliferation, differentiation, and matrix production. These studies can help identify specific mechanical cues that promote healthy ligament function and prevent degeneration.125
Gene expression analysis in ACL tissues exposed to mechanical stress has provided insights into the molecular responses that underpin ligament health. For example, studies have identified specific genes that are upregulated or downregulated in response to mechanical loading, providing targets for potential therapeutic intervention. By modulating the expression of these genes, it may be possible to enhance the ACL’s resilience to mechanical stress and reduce the risk of injury.126
In summary, a narrower femoral notch is a significant anatomical factor associated with an increased risk of ACL injury. MRI and cadaveric studies have demonstrated that limited space for the ACL within the notch increases its susceptibility to impingement during high-stress activities, potentially leading to rupture. Understanding the biomechanical and developmental aspects of notch width can inform the development of targeted prevention and intervention strategies, ultimately enhancing the safety and performance of individuals at risk for ACL injuries. Molecular biophysics provides valuable insights into the underlying mechanisms of ACL damage, including the role of collagen degradation, mechanotransduction pathways, MMP activity, oxidative stress, and mechanobiology, paving the way for innovative interventions to enhance ligament resilience and prevent injuries.
2. Tibial Slope
An increased posterior tibial slope has been identified as a significant risk factor for ACL injuries. The tibial slope refers to the angle of the tibial plateau relative to the long axis of the tibia. When the posterior tibial slope is steeper, it creates a biomechanical environment that facilitates greater anterior tibial translation, especially under load, thereby increasing strain on the ACL.127
Biomechanical modeling and radiographic studies provide crucial insights into how tibial slope affects knee mechanics and ACL strain. Biomechanical models simulate the forces and movements within the knee joint, allowing researchers to predict how different tibial slope angles influence the stress distribution on the ACL. These models have consistently shown that a steeper posterior tibial slope results in increased anterior tibial translation during weight-bearing activities, such as running, jumping, and cutting maneuvers. This anterior movement of the tibia relative to the femur places additional tension on the ACL, heightening the risk of injury.128
Radiographic studies, including X-rays and MRI, allow for precise measurement of the tibial slope and provide empirical data on its impact on ACL strain. By comparing the tibial slopes of individuals with and without ACL injuries, researchers have found a strong correlation between a steeper slope and a higher incidence of ACL tears. These imaging techniques also help in assessing other anatomical variations that may contribute to ACL vulnerability, such as femoral notch width and overall knee alignment.129
The increased anterior tibial translation associated with a steeper tibial slope can be particularly problematic during dynamic movements that involve sudden changes in direction or deceleration. For example, when an athlete lands from a jump or makes a rapid pivot, the forces exerted on the knee can cause the tibia to slide forward excessively if the posterior tibial slope is steep. This excessive anterior translation strains the ACL, making it more susceptible to tears.130
Molecular biophysics provides deeper insights into how tibial slope affects ACL mechanics at a microscopic level. The stress and strain on the ACL due to increased anterior tibial translation can lead to microstructural changes within the collagen matrix. The ACL is primarily composed of type I collagen, a protein that provides tensile strength and structural integrity. Repeated strain on the ligament can cause collagen fibrils to undergo deformation, affecting their alignment and cross-linking patterns. This degradation at the molecular level weakens the overall structure of the ACL, making it more susceptible to tears.131
Moreover, molecular studies have shown that the response of ACL cells, known as fibroblasts, to mechanical stress involves complex signaling pathways. Mechanical loading triggers mechanotransduction processes, where mechanical stimuli are converted into biochemical signals within the cells. This process involves integrins, which are transmembrane receptors that connect the extracellular matrix (ECM) to the cytoskeleton. When integrins detect mechanical strain, they activate intracellular signaling cascades that can lead to changes in gene expression and protein synthesis.132
One crucial signaling pathway activated by mechanical stress is the MAPK/ERK pathway. This pathway is involved in the cellular response to a variety of stressors, including mechanical load, and plays a role in regulating cell proliferation, differentiation, and apoptosis. In the context of increased tibial slope, chronic activation of stress-responsive pathways can lead to an imbalance between ECM synthesis and degradation, further compromising the ligament’s integrity.133
Research into the role of matrix metalloproteinases (MMPs) in ACL degeneration provides additional molecular insights. MMPs are enzymes that break down collagen and other ECM components. Under normal conditions, MMP activity is tightly regulated to maintain tissue homeostasis. However, excessive mechanical stress and increased anterior tibial translation can upregulate MMP expression, leading to increased collagen degradation and weakening of the ACL. Understanding the regulation of MMPs in response to mechanical stress could inform therapeutic strategies to mitigate ACL damage.134
Another area of interest in molecular biophysics is the role of oxidative stress in ACL injuries. Mechanical stress and increased anterior tibial translation can generate reactive oxygen species (ROS), which are chemically reactive molecules containing oxygen. ROS can cause oxidative damage to cellular components, including lipids, proteins, and DNA. In the ACL, oxidative stress can impair fibroblast function and promote ECM degradation. Antioxidant therapies aimed at reducing oxidative stress could potentially protect the ACL from damage associated with increased tibial slope.135
Furthermore, the role of mechanobiology in ACL health is an essential aspect of molecular biophysics. Mechanobiology explores how mechanical forces influence cellular behavior and tissue remodeling. In the case of the ACL, mechanobiology studies can reveal how mechanical loading patterns affect cellular activities such as proliferation, differentiation, and matrix production. These studies can help identify specific mechanical cues that promote healthy ligament function and prevent degeneration.136
Gene expression analysis in ACL tissues exposed to mechanical stress has provided insights into the molecular responses that underpin ligament health. For example, studies have identified specific genes that are upregulated or downregulated in response to mechanical loading, providing targets for potential therapeutic intervention. By modulating the expression of these genes, it may be possible to enhance the ACL’s resilience to mechanical stress and reduce the risk of injury.137
Additionally, advanced imaging techniques and computational modeling play a crucial role in understanding the impact of tibial slope on ACL strain. Three-dimensional (3D) imaging technologies, such as MRI and CT scans, allow for precise visualization of the tibial slope and its relationship with other knee structures. Computational models can integrate these imaging data to simulate the biomechanical environment of the knee joint, predicting how variations in tibial slope influence ACL loading. These models can also be used to evaluate the effectiveness of different surgical interventions, such as tibial slope modification, in reducing ACL injury risk.138
In summary, an increased posterior tibial slope is a significant risk factor for ACL injuries. Biomechanical modeling and radiographic studies have shown that a steeper slope facilitates greater anterior tibial translation under load, thereby increasing ACL strain. Understanding the biomechanical and molecular implications of tibial slope variations can inform the development of targeted prevention and intervention strategies.139 Molecular biophysics provides valuable insights into the underlying mechanisms of ACL damage, including the role of collagen degradation, mechanotransduction pathways, MMP activity, oxidative stress, and mechanobiology. By combining advanced imaging techniques, computational modeling, and molecular studies, researchers can develop innovative approaches to enhance ligament resilience and prevent injuries.140
To expand further, molecular biophysics delves into the intricate details of how these mechanical forces impact the microstructure and molecular composition of the ACL. At the nanoscale, the collagen fibrils within the ACL are composed of tropocollagen molecules that assemble into fibrils with specific cross-linking patterns. These cross-links are critical for the mechanical strength and integrity of the collagen network. When subjected to mechanical stress, such as that induced by a steep tibial slope, these cross-links can become disrupted, leading to weakened fibril structure and reduced overall ligament strength.141
Additionally, the role of proteoglycans and other glycoproteins in the ACL’s extracellular matrix is crucial. Proteoglycans, such as decorin and aggrecan, interact with collagen fibrils and contribute to the viscoelastic properties of the ligament. Mechanical loading can alter the synthesis and degradation of these proteoglycans, affecting the hydration and mechanical properties of the ACL. Understanding these molecular interactions can provide insights into how to preserve or restore ligament function under mechanical stress.142
Another critical aspect of molecular biophysics is the study of mechanosensitive ion channels and their role in ACL function. Ion channels such as Piezo1 and TRPV4 respond to mechanical stimuli by altering ion flux across the cell membrane, leading to changes in intracellular signaling pathways.143 These mechanosensitive channels play a role in cellular responses to mechanical stress, including the regulation of cytoskeletal dynamics and gene expression. Investigating how these channels contribute to ACL mechanotransduction could reveal new targets for therapeutic intervention to enhance ligament resilience.144
Epigenetic modifications in response to mechanical stress also represent an emerging area of research in molecular biophysics. Mechanical forces can lead to changes in DNA methylation, histone modifications, and non-coding RNA expression, which in turn affect gene expression and cellular behavior. Epigenetic regulation plays a role in the adaptive responses of ACL fibroblasts to mechanical loading. Understanding these epigenetic mechanisms could inform the development of strategies to promote adaptive responses and prevent maladaptive changes that increase the risk of injury.145
Furthermore, molecular biophysics research explores the impact of mechanical stress on mitochondrial function and energy metabolism in ACL cells. Mitochondria are critical for providing the energy required for cellular processes, including the synthesis of extracellular matrix components and the maintenance of cellular homeostasis.146 Mechanical loading can influence mitochondrial dynamics, affecting their biogenesis, fission, fusion, and function. Disruptions in mitochondrial function can lead to altered cellular energy metabolism, increased production of ROS, and impaired cellular responses to mechanical stress.147
By integrating these molecular insights with biomechanical and clinical data, researchers can develop comprehensive models that predict ACL injury risk based on tibial slope and other anatomical factors. These models can be used to design personalized prevention and treatment strategies that address the specific molecular and biomechanical mechanisms underlying ACL injuries. For example, interventions that target specific molecular pathways involved in collagen synthesis, mechanotransduction, or oxidative stress could be developed to enhance ligament resilience and reduce the risk of injury.148
In summary, an increased posterior tibial slope is a significant risk factor for ACL injuries. Biomechanical modeling and radiographic studies have shown that a steeper slope facilitates greater anterior tibial translation under load, thereby increasing ACL strain. Molecular biophysics provides valuable insights into the underlying mechanisms of ACL damage, including the role of collagen degradation, proteoglycan interactions, mechanosensitive ion channels, epigenetic modifications, and mitochondrial function. By combining advanced imaging techniques, computational modeling, and molecular studies, researchers can develop innovative approaches to enhance ligament resilience, prevent injuries, and inform personalized prevention and treatment strategies.
3. ACL Geometry
Variations in ACL size, shape, and insertion points can significantly affect its mechanical properties and risk of injury. The anatomical characteristics of the ACL are critical in determining its ability to resist forces and maintain knee stability. Studies using MRI and 3D reconstruction techniques have demonstrated that these variations can influence the ligament’s biomechanical performance and susceptibility to injury.149
The ACL’s size, including its length and cross-sectional area, plays a vital role in its mechanical strength. A larger cross-sectional area generally indicates a stronger ligament capable of withstanding greater forces. Conversely, a smaller or thinner ACL may be less resilient to mechanical stress, making it more prone to tears. MRI studies have provided detailed images of the ACL’s size in different individuals, showing considerable variation that correlates with injury risk. For instance, athletes with smaller ACL cross-sectional areas have been found to have a higher incidence of ACL injuries.150
The shape of the ACL, including its curvature and orientation within the knee joint, also affects its mechanical properties. An ACL that is more curved or has an irregular shape may experience uneven stress distribution during dynamic movements. This uneven stress can lead to localized areas of higher strain, increasing the risk of microtears and eventual rupture. 3D reconstruction techniques allow for precise mapping of the ACL’s shape, providing insights into how these geometric variations impact its function and injury susceptibility.151
Insertion points, or the locations where the ACL attaches to the femur and tibia, are another crucial factor influencing the ligament’s mechanical properties. Variations in the position and angle of these insertion points can alter the ACL’s leverage and the forces it experiences during knee movements. For example, an ACL with insertion points that are more anteriorly or posteriorly positioned may be subjected to different tensile forces compared to one with centrally located insertions. These differences can affect the ligament’s ability to resist anterior tibial translation and rotational forces, key factors in ACL stability and injury prevention.152
Molecular biophysics provides deeper insights into how these geometric variations impact the ACL at the microscopic and molecular levels. The ACL is primarily composed of type I collagen, a protein that forms a triple-helix structure, providing tensile strength and flexibility. Variations in the size, shape, and insertion points of the ACL can influence the alignment and density of collagen fibers, affecting the ligament’s overall mechanical properties. For example, a smaller ACL with tightly packed collagen fibers may have different viscoelastic properties compared to a larger ligament with a more loosely organized collagen matrix.153
Moreover, the interaction between collagen fibers and other extracellular matrix (ECM) components, such as proteoglycans and elastin, can be influenced by ACL geometry. Proteoglycans, which are large molecules that attract water, contribute to the ligament’s compressive strength and ability to resist deformation. Elastin provides elasticity, allowing the ligament to stretch and return to its original shape. Variations in ACL geometry can affect the distribution and interaction of these ECM components, impacting the ligament’s mechanical behavior under load.154
The cellular response to mechanical stress within the ACL is also modulated by its geometry. ACL fibroblasts, the cells responsible for producing and maintaining the ECM, respond to mechanical loading through mechanotransduction pathways. These pathways involve integrins, which are transmembrane proteins that connect the ECM to the cytoskeleton. When mechanical forces are applied to the ACL, integrins transmit signals into the cell, leading to changes in gene expression and protein synthesis. Variations in ACL geometry can influence the distribution of mechanical forces across the ligament, affecting the activation of mechanotransduction pathways and the cellular response to stress.155
One important mechanotransduction pathway involves the activation of focal adhesion kinase (FAK), a protein that plays a key role in cell adhesion and signal transduction. FAK is activated in response to mechanical stress and helps regulate cell survival, proliferation, and ECM production. Differences in ACL geometry can lead to variations in FAK activation, influencing the ligament’s ability to adapt to mechanical stress and maintain structural integrity.156
Molecular studies have also shown that variations in ACL geometry can affect the expression of matrix metalloproteinases (MMPs), enzymes that degrade collagen and other ECM components. MMPs are involved in the remodeling and repair of the ligament in response to mechanical stress. However, excessive MMP activity can lead to increased collagen degradation and weakening of the ACL. Understanding how ACL geometry influences MMP expression and activity could inform therapeutic strategies to enhance ligament resilience and prevent injuries.157
Additionally, the role of growth factors in ACL maintenance and repair is influenced by the ligament’s geometry. Growth factors such as transforming growth factor-beta (TGF-β) and insulin-like growth factor-1 (IGF-1) play crucial roles in promoting collagen synthesis and regulating cellular responses to mechanical stress. Variations in ACL size, shape, and insertion points can affect the distribution and activity of these growth factors, impacting the ligament’s ability to repair and adapt to mechanical loading.158
Advanced imaging techniques, such as high-resolution MRI and 3D ultrasound, combined with computational modeling, provide valuable tools for studying ACL geometry and its impact on injury risk. These techniques allow for precise measurement of the ligament’s size, shape, and insertion points, as well as detailed analysis of its internal structure and mechanical properties. Computational models can simulate the biomechanical environment of the knee joint, predicting how variations in ACL geometry influence stress distribution and injury risk.159
Furthermore, understanding the molecular composition of the ACL and how it changes in response to mechanical stress is crucial for developing targeted interventions. The ACL contains various types of collagen (primarily type I but also types III and V) and non-collagenous proteins that contribute to its mechanical properties.160 Variations in the relative abundance and organization of these proteins can influence the ligament’s strength and elasticity. For example, type III collagen is more elastic but less tensile than type I, and an increased proportion of type III collagen could impact the ligament’s ability to withstand tensile forces.161
Research into the role of small leucine-rich proteoglycans (SLRPs) in the ACL, such as decorin and biglycan, has shown that these molecules interact with collagen fibers to regulate fibrillogenesis and ECM organization. Variations in the expression of SLRPs can affect the structural integrity and mechanical properties of the ACL. For instance, decorin binds to collagen fibrils and influences their diameter and spacing, which in turn affects the ligament’s mechanical strength.162
The study of epigenetic modifications in ACL fibroblasts is another area of molecular biophysics that can provide insights into how genetic and environmental factors influence ligament health. Epigenetic changes, such as DNA methylation and histone modification, can regulate gene expression in response to mechanical stress. Understanding these epigenetic mechanisms can help identify how variations in ACL geometry might predispose individuals to injuries and guide the development of personalized prevention and treatment strategies.163
The investigation of biomechanical properties at the nanoscale using atomic force microscopy (AFM) can further enhance our understanding of ACL mechanics. AFM can measure the stiffness and viscoelastic properties of individual collagen fibrils and other ECM components, providing detailed information about how molecular structure relates to mechanical function. These measurements can help elucidate how variations in ACL geometry affect the nanoscale properties of the ligament, which in turn influence its macroscopic behawior.164
Finally, advances in tissue engineering and regenerative medicine hold promise for addressing variations in ACL geometry. Techniques such as 3D bioprinting and scaffold-based approaches aim to create ligament constructs that mimic the native ACL’s geometry and mechanical properties. By understanding the molecular and biomechanical principles that govern ACL function, researchers can design scaffolds and bioprinted tissues that promote proper cell alignment, ECM organization, and mechanical strength, potentially improving outcomes for ACL reconstruction and repair.165
In summary, variations in ACL size, shape, and insertion points significantly affect its mechanical properties and risk of injury. Studies using MRI and 3D reconstruction techniques have shown that these anatomical variations can influence the ligament’s ability to resist forces. Molecular biophysics provides valuable insights into the underlying mechanisms of ACL damage, including the role of collagen organization, ECM interactions, mechanotransduction pathways, MMP activity, growth factor signaling, and epigenetic regulation.166 By combining advanced imaging techniques, computational modeling, and molecular studies, researchers can develop innovative approaches to enhance ligament resilience, prevent injuries, and inform personalized prevention and treatment strategies. Understanding the interplay between ACL geometry, biomechanics, and molecular mechanisms is essential for improving athlete safety and performance.
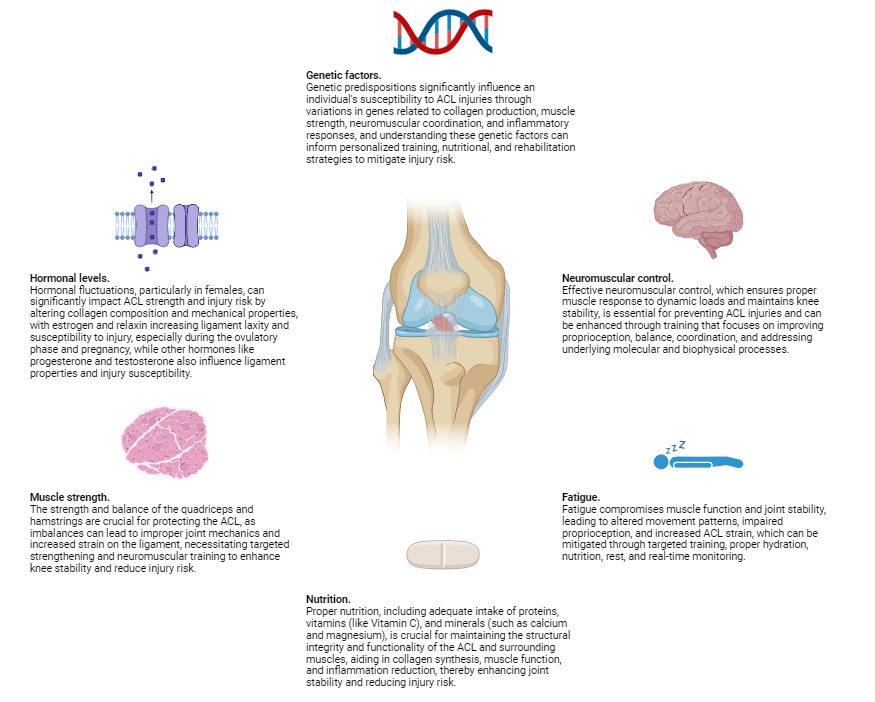
Physiological Factors
Physiological factors play a crucial role in determining the risk of ACL injuries. These factors encompass a wide range of elements, including hormonal levels, muscle strength, neuromuscular control, and fatigue. Hormonal fluctuations, particularly in females, can affect ligament laxity and susceptibility to injury, as variations in estrogen levels have been linked to changes in the mechanical properties of the ACL.167 Muscle strength is another critical component; imbalances or weaknesses in the muscles surrounding the knee can lead to inadequate support and increased strain on the ligament. Neuromuscular control, which involves the coordination and timing of muscle activations, is essential for maintaining joint stability during dynamic movements. Poor neuromuscular control can result in improper joint alignment and increased risk of injury. Additionally, fatigue can significantly impair both muscle function and neuromuscular control, further heightening the likelihood of ACL injuries.168 As physical activity continues, the muscles may become less effective at stabilizing the knee, leading to greater reliance on the ACL and increased risk of damage.169
From the perspective of molecular biophysics, the structural integrity of the ACL is influenced by the molecular composition and organization of its constituent collagen fibers. Collagen, the primary structural protein in the ACL, exhibits unique biomechanical properties that contribute to the ligament’s strength and elasticity. The cross-linking between collagen molecules plays a vital role in maintaining the tensile strength and resistance of the ACL to mechanical forces. Disruptions in these molecular interactions, whether due to genetic factors, biochemical imbalances, or hormonal influences, can compromise the ligament’s ability to withstand stress.170
Additionally, molecular biophysics explores the interactions between cellular components and the extracellular matrix, which can affect the overall health and repair mechanisms of the ACL.171 For instance, the activity of fibroblasts, the cells responsible for collagen synthesis and repair, is crucial for maintaining ligament integrity. Any alterations in the signaling pathways that regulate fibroblast function can impact collagen production and, consequently, the resilience of the ACL. Understanding these molecular-level dynamics provides deeper insights into how physiological factors such as hormonal changes, muscle strength, and neuromuscular control can influence the risk of ACL injuries.172
Each of these aspects can influence the mechanical properties of the ACL and its ability to withstand the stresses encountered during physical activities, making them critical considerations in both the prevention and rehabilitation of ACL injuries (Table 4.). By integrating knowledge from molecular biophysics with physiological factors, we can develop more effective strategies for injury prevention, early detection of susceptibility, and targeted rehabilitation protocols tailored to the individual needs of patients (Figure 2).
1. Hormonal Levels
Hormonal fluctuations, particularly in females, have been shown to significantly impact ACL strength and injury risk. Estrogen and relaxin, hormones that increase in concentration during certain phases of the menstrual cycle, can reduce the tensile strength of the ACL by affecting the composition and mechanical properties of the collagen fibers.173 Studies have demonstrated that during the ovulatory phase, when estrogen levels peak, there is an increased laxity in ligaments, including the ACL, making them more susceptible to injury. This increased laxity is attributed to estrogen’s influence on the collagen structure, leading to a temporary decrease in the ligament’s stiffness and strength. Moreover, relaxin, which increases during pregnancy, can also contribute to ligamentous laxity and a higher risk of ACL tears.174
Relaxin is known to remodel the extracellular matrix and increase the elasticity of connective tissues, which, while beneficial for childbirth, can compromise the stability of the knee joint. This hormone induces the breakdown of collagen fibers, resulting in a softer and more pliable ligament structure. During pregnancy, the elevated levels of relaxin can lead to a generalized increase in joint laxity, making pregnant women more prone to ACL injuries, particularly if they engage in high-impact or sudden directional-change activities.175
Beyond estrogen and relaxin, other hormones such as progesterone and testosterone may also play roles in ligament properties and injury susceptibility. Progesterone, which fluctuates during the menstrual cycle, may have protective effects against ligament injuries by counteracting some of the laxity induced by estrogen. Conversely, lower levels of testosterone in females compared to males might contribute to differences in muscle strength and joint stability, further influencing ACL injury risk.176
From a molecular biophysics perspective, these hormonal influences can be understood in terms of their impact on the molecular structure and behavior of the ACL’s collagen fibers. Collagen, the primary structural protein in ligaments, consists of triple helices that form robust fibrils through intermolecular cross-linking.177 Hormones like estrogen and relaxin can alter the synthesis and degradation of collagen, affecting its density, cross-linking, and alignment. For instance, estrogen may interfere with the cross-linking process, leading to a reduction in the mechanical integrity of collagen fibrils. Relaxin, on the other hand, enhances matrix metalloproteinase (MMP) activity, enzymes that degrade collagen and other extracellular matrix components, resulting in a more compliant ligament.178
Furthermore, molecular biophysics studies suggest that hormonal fluctuations can influence the expression and activity of integrins and other cell-adhesion molecules in fibroblasts, the cells responsible for collagen production and maintenance.179 These molecular changes can lead to variations in the mechanical properties of the ACL over the menstrual cycle and pregnancy. For example, integrins play a critical role in transmitting mechanical signals from the extracellular matrix to the intracellular cytoskeleton, influencing cell behavior and tissue remodeling. Hormonal changes can modulate these signaling pathways, altering fibroblast activity and collagen turnover rates, thereby impacting the structural integrity of the ACL.180
Biophysics also explores the biomechanical environment of the ACL at the tissue level, examining how forces and mechanical stress are distributed across the ligament during various activities. Hormonal variations can affect the viscoelastic properties of the ACL, which describes its ability to absorb and dissipate energy. Estrogen, for instance, has been shown to decrease the viscoelasticity of ligaments, making them more prone to microtears under repetitive stress. This reduction in viscoelasticity can compromise the ligament’s ability to recover from deformations, increasing the risk of a complete tear during sudden movements or impacts.181
Advanced biophysical techniques, such as atomic force microscopy (AFM) and magnetic resonance elastography (MRE), allow researchers to measure the nanoscale mechanical properties of ACL tissue and observe changes induced by hormonal fluctuations. These techniques provide detailed insights into how the structural organization of collagen and other extracellular matrix components is altered under different hormonal conditions. For instance, AFM can be used to assess the stiffness and adhesion properties of individual collagen fibrils, revealing how estrogen or relaxin affects their mechanical behawior.182
Understanding the molecular biophysics behind hormonal effects on the ACL can also aid in developing advanced biomaterials and therapeutic interventions. For instance, targeted delivery of hormone-modulating drugs or the use of tissue engineering approaches to reinforce the ACL during high-risk hormonal phases could be potential strategies for preventing injuries. Additionally, personalized medicine approaches that consider an individual’s hormonal profile and molecular responses could lead to more effective prevention and treatment of ACL injuries.183
Incorporating this hormonal and molecular knowledge into training and rehabilitation programs can lead to more personalized and effective approaches, reducing the incidence of ACL injuries and improving recovery outcomes for those affected. By leveraging insights from molecular biophysics, medical professionals can better understand the complex interplay between hormones and ligament health, ultimately enhancing athletic performance and reducing injury risks. This integrated approach underscores the importance of considering both macroscopic and microscopic factors in managing ACL health and developing comprehensive strategies for injury prevention and rehabilitation.
2. Muscle Strength
The strength and balance of the muscles surrounding the knee joint are critical for protecting the ACL. The quadriceps and hamstrings play pivotal roles in stabilizing the knee. Weakness or imbalances in these muscles can lead to improper joint mechanics, increasing the strain on the ACL. Strong hamstrings, for example, help counteract the anterior translation of the tibia, a major factor in ACL stress.184 The hamstrings work by pulling the tibia backward, thereby reducing the forward motion that puts strain on the ACL. In contrast, dominant quadriceps can exacerbate this translation if not balanced by adequate hamstring strength. When the quadriceps are overly strong relative to the hamstrings, they can pull the tibia forward excessively during activities like jumping, landing, and cutting, which increases the risk of ACL injuries.185
To mitigate this risk, strengthening these muscles through targeted exercises can enhance knee stability and reduce ACL injury risk. Exercises that focus on hamstring strengthening, such as leg curls, deadlifts, and Nordic hamstring curls, are particularly beneficial. These exercises not only increase hamstring strength but also improve the muscle’s ability to control and decelerate the lower leg during dynamic movements. Similarly, quadriceps-strengthening exercises, like squats and lunges, should be performed with an emphasis on achieving balanced strength and coordination between the front and back of the thigh.186
Furthermore, it’s essential to incorporate neuromuscular training into strength programs. This type of training enhances proprioception—the body’s ability to sense the position and movement of the joints—which is crucial for maintaining knee stability. Neuromuscular exercises might include balance drills, plyometrics, and agility exercises that simulate real-life sports movements. These drills help improve the timing and coordination of muscle contractions, ensuring that the quadriceps and hamstrings work together effectively to stabilize the knee.187
In addition to traditional strength training and neuromuscular exercises, incorporating functional training that mimics sport-specific activities can further reduce ACL injury risk. Functional training involves performing exercises that replicate the movements commonly encountered in sports, such as cutting, pivoting, and jumping. This type of training helps athletes develop the strength and coordination needed to perform these movements safely, reducing the likelihood of placing excessive strain on the ACL.188
Moreover, flexibility and mobility exercises should not be overlooked. Tight muscles can alter joint mechanics and contribute to improper movement patterns. Stretching routines that target the hamstrings, quadriceps, and hip flexors can help maintain optimal muscle length and joint range of motion, which are essential for proper knee function.189
From a molecular biophysics perspective, muscle contractions and the resulting mechanical forces exerted on the ACL can be examined at the cellular and molecular levels. Muscle fibers generate force through the interaction of actin and myosin filaments within sarcomeres, the fundamental contractile units of muscle cells. The efficiency and strength of these interactions are influenced by the biochemical environment and the structural integrity of the muscle tissue.190
Biophysical studies have shown that mechanical stress on the ACL is transmitted through the muscle-tendon complex to the bone-ligament interface, where cellular mechanotransduction occurs. Mechanotransduction refers to the process by which cells sense and respond to mechanical stimuli.191 In the context of the ACL, fibroblasts—the primary cells in the ligament—respond to mechanical loading by altering their production of extracellular matrix components, such as collagen. This adaptive response helps maintain the structural integrity of the ligament under varying mechanical demands.192
Additionally, molecular biophysics research explores the role of integrins and other mechanosensitive proteins in mediating the cellular response to mechanical stress. Integrins are transmembrane receptors that connect the extracellular matrix to the intracellular cytoskeleton, facilitating the transmission of mechanical signals.193 When muscles contract and generate force, these signals can prompt changes in gene expression and protein synthesis within fibroblasts, enhancing the ligament’s resilience and repair capabilities.194
Advanced imaging techniques, such as atomic force microscopy (AFM) and magnetic resonance elastography (MRE), enable researchers to visualize and quantify the nanoscale mechanical properties of muscle and ligament tissues. These techniques provide insights into how changes in muscle strength and coordination affect the microscopic structure and mechanical behavior of the ACL. For example, AFM can measure the stiffness of individual collagen fibrils, revealing how training and conditioning regimens might influence the mechanical properties of the ACL at a molecular level.195
Molecular biophysics also delves into the understanding of how biochemical signals regulate muscle and ligament adaptations. For instance, the activation of specific signaling pathways, such as the MAPK/ERK pathway in response to mechanical stress, can lead to increased synthesis of collagen and other extracellular matrix proteins, fortifying the ACL.196 The interplay between mechanical and biochemical signals is crucial for the adaptation of musculoskeletal tissues to physical training, emphasizing the importance of balanced muscle conditioning to protect the ACL.197
Moreover, understanding the molecular basis of muscle-ligament interactions can guide the development of therapeutic interventions. For example, biomaterials engineered to mimic the natural extracellular matrix could be used in regenerative medicine to repair damaged ACL tissue. These materials could be designed to release growth factors and other bioactive molecules that promote the proliferation and differentiation of fibroblasts, enhancing the repair proces.198
Incorporating this hormonal and molecular knowledge into training and rehabilitation programs can lead to more personalized and effective approaches, reducing the incidence of ACL injuries and improving recovery outcomes for those affected.199 By leveraging insights from molecular biophysics, medical professionals can better understand the complex interplay between muscles and ligaments, ultimately enhancing athletic performance and reducing injury risks. This integrated approach underscores the importance of considering both macroscopic and microscopic factors in managing ACL health and developing comprehensive strategies for injury prevention and rehabilitation.
3. Neuromuscular Control
Effective neuromuscular control ensures that the muscles around the knee joint respond appropriately to dynamic loads and maintain joint stability. This control is essential for the synchronization and timing of muscle activations, which help stabilize the knee during various movements.200 Poor neuromuscular control, often due to inadequate proprioception or coordination, can result in delayed muscle activation and improper joint alignment during movements. Such deficiencies can cause the knee to move in an uncontrolled manner, increasing the risk of excessive strain on the ACL. This can significantly increase the risk of ACL injuries, especially during activities involving sudden stops, jumps, or changes in direction. These high-risk movements require precise and timely muscle responses to maintain joint stability and prevent ligamentous injuries.201
Training programs that focus on improving proprioception, balance, and coordination can enhance neuromuscular control and reduce injury risk. Proprioceptive training involves exercises that improve the body’s ability to sense the position and movement of the joints, such as balancing on one leg, using balance boards, or performing exercises on unstable surfaces. These exercises stimulate the sensory receptors in the muscles and joints, enhancing the brain’s ability to process and respond to proprioceptive input, thereby improving joint stability.202
Balance training is another critical component, involving exercises that challenge the body’s ability to maintain equilibrium. This can include single-leg stands, dynamic balance activities like hopping or jumping onto a soft surface, and the use of tools like balance discs or Bosu balls. These exercises improve the muscles’ ability to make rapid adjustments to maintain stability, which is crucial for protecting the ACL during dynamic movements.203
Coordination training aims to enhance the efficient and smooth execution of movements. Drills that require precise timing and control, such as agility ladders, cone drills, and plyometric exercises, can improve neuromuscular coordination. These activities help train the nervous system to activate muscles in the correct sequence and with appropriate force, reducing the likelihood of improper joint alignment and excessive strain on the ACL.204
From a molecular biophysics perspective, neuromuscular control can be understood in terms of the biophysical processes that underlie muscle contraction and neuronal signaling. Effective neuromuscular control relies on the rapid transmission of electrical signals from the brain to the muscles, mediated by motor neurons. These signals prompt the release of calcium ions within muscle fibers, initiating the interaction between actin and myosin filaments that produce muscle contraction. Any delays or disruptions in this signaling pathway can impair muscle activation timing, compromising joint stability.205
At the cellular level, the strength and coordination of muscle contractions depend on the structural integrity of the sarcomeres and the efficient function of the neuromuscular junctions. Training that enhances neuromuscular control can promote adaptations such as increased synaptic efficiency and enhanced calcium handling within muscle cells, leading to more precise and powerful muscle contractions.206
Incorporating molecular biology further deepens our understanding of neuromuscular control by exploring the genetic and biochemical pathways that regulate muscle function and neural connectivity. Molecular biology reveals that muscle fiber types, determined by the expression of specific myosin heavy chain genes, play a crucial role in muscle performance and fatigue resistance. Training can induce shifts in muscle fiber type composition, enhancing the proportion of fast-twitch or slow-twitch fibers depending on the demands of the activity, thereby optimizing the muscle’s ability to respond to rapid or sustained loadsn.207
Additionally, molecular biology highlights the role of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), in promoting the growth and maintenance of neuromuscular connections. Exercise has been shown to upregulate the expression of these neurotrophic factors, facilitating synaptic plasticity and enhancing the communication between neurons and muscle fibers. This improved neural connectivity is critical for precise motor control and rapid response to dynamic movements.208
Genomic studies also provide insights into the individual variability in neuromuscular control and injury risk. Polymorphisms in genes associated with collagen synthesis, muscle repair, and inflammation can influence an individual’s susceptibility to ACL injuries. For instance, variations in the COL1A1 gene, which encodes a key component of collagen, can affect ligament strength and elasticity.209 Understanding these genetic predispositions can inform personalized training and rehabilitation programs that account for an individual’s unique molecular profile.
Epigenetic modifications, such as DNA methylation and histone acetylation, also play a role in regulating gene expression in response to mechanical stress and training. These epigenetic changes can modulate the activity of genes involved in muscle hypertrophy, collagen turnover, and inflammatory responses, thereby influencing the adaptation of the neuromuscular system to exercise.210 Targeting these epigenetic pathways through specific training protocols or pharmacological interventions could enhance neuromuscular control and reduce the risk of ACL injuries.
Advanced imaging techniques, such as atomic force microscopy (AFM) and magnetic resonance elastography (MRE), enable researchers to visualize and quantify the nanoscale mechanical properties of muscle and ligament tissues. These techniques provide insights into how changes in muscle strength and coordination affect the microscopic structure and mechanical behavior of the ACL. For example, AFM can measure the stiffness of individual collagen fibrils, revealing how training and conditioning regimens might influence the mechanical properties of the ACL at a molecular level.211 MRE can assess the viscoelastic properties of muscle and ligament tissues in vivo, providing a comprehensive understanding of how these tissues respond to mechanical stress and training.
Moreover, molecular biophysics research explores the role of mechanosensitive ion channels in neuromuscular control. These channels, such as Piezo1 and Piezo2, respond to mechanical stimuli by allowing ions to flow into cells, triggering downstream signaling pathways that regulate cellular responses to mechanical stress.212 Understanding how these channels contribute to neuromuscular control can inform the development of novel interventions to enhance muscle function and joint stability.
Incorporating these biophysical and molecular biology insights into training and rehabilitation programs can lead to more effective strategies for enhancing neuromuscular control and reducing ACL injury risk. By understanding the underlying molecular and cellular mechanisms, trainers and therapists can design exercises that specifically target the critical aspects of neuromuscular function. This holistic approach can improve athletic performance, reduce injury incidence, and contribute to more effective rehabilitation protocols for those recovering from ACL injuries.
4. Fatigue
Fatigue has a profound impact on muscle function and joint stability. As muscles tire, their ability to support and stabilize the knee diminishes, leading to altered movement patterns and increased strain on the ACL. When muscles are fatigued, they can no longer generate the same level of force or respond as quickly to changes in movement, resulting in compromised joint stability. This reduction in force production and delayed response times means that the muscles are less effective at controlling the knee’s movement, especially during high-intensity activities that involve rapid direction changes, jumping, and landing.213
Fatigued muscles also exhibit altered proprioception, which can impair the body’s ability to sense joint position and movement. This impairment can lead to incorrect joint positioning and increased susceptibility to injury. For instance, fatigue-induced changes in biomechanics, such as increased knee valgus (inward collapse of the knee) and internal rotation, are associated with a higher risk of ACL injuries. These changes can occur because the neuromuscular system becomes less efficient at maintaining proper alignment and coordination of the lower limb, which places additional stress on the ACL.214
Moreover, fatigue affects not only the muscles around the knee but also the entire kinetic chain, including the hip and ankle. When these proximal and distal joints are not adequately stabilized, it further increases the likelihood of abnormal knee mechanics. Fatigue can lead to a cascade of compensatory movements and muscle activation patterns that exacerbate the strain on the ACL.215 For example, weakened hip muscles might fail to control hip adduction and internal rotation, contributing to excessive knee valgus and increasing ACL loading.
From a molecular biophysics perspective, fatigue influences muscle function at the cellular and molecular levels. During prolonged or intense exercise, the accumulation of metabolic byproducts such as lactic acid and the depletion of energy stores can impair the contractile function of muscle fibers. This metabolic stress affects the interaction between actin and myosin filaments within the sarcomeres, reducing the muscles’ ability to generate force and contract efficiently.216 Additionally, changes in ion concentrations, particularly calcium, can disrupt excitation-contraction coupling, further diminishing muscle performance.
Molecular biology insights reveal that fatigue also induces changes in gene expression and protein synthesis within muscle cells. For example, the expression of fatigue-related genes and the activation of signaling pathways involved in muscle repair and adaptation are upregulated in response to prolonged exercise. These molecular adaptations are essential for enhancing muscular endurance and resistance to fatigue over time.217 Training programs that focus on improving these aspects of muscular endurance can help mitigate the adverse effects of fatigue on joint stability and ACL injury risk.
In the realm of molecular biophysics, the impact of fatigue on muscle and joint function is further elucidated through the study of protein dynamics and cellular signaling pathways. Fatigue leads to alterations in the structure and function of key proteins involved in muscle contraction.218 For example, the troponin-tropomyosin complex, which regulates the interaction between actin and myosin, can be affected by changes in pH and ion concentrations during fatigue. This can result in a reduced sensitivity of the contractile apparatus to calcium, thereby impairing muscle contraction efficiency.219
The role of ion channels and transporters in maintaining cellular homeostasis during muscle activity is also critical. During fatigue, the efficiency of ion pumps such as the sodium-potassium ATPase and calcium ATPase can be compromised, leading to disrupted ion gradients across the muscle cell membrane. This disruption can affect the excitability of muscle cells and the propagation of action potentials, which are essential for coordinated muscle contractions.218
Fatigue also influences the mechanical properties of the extracellular matrix (ECM) within muscle tissue. The ECM provides structural support and transmits mechanical signals to muscle cells. Under conditions of fatigue, the composition and stiffness of the ECM can change, affecting the mechanical environment of muscle cells. These changes can influence the behavior of mechanosensitive proteins such as integrins, which mediate the cell’s response to mechanical stress. Altered signaling through these proteins can impact muscle repair and adaptation processes.220
Advanced imaging techniques, such as atomic force microscopy (AFM) and optical tweezers, allow researchers to study the mechanical properties of muscle proteins and cells at the nanoscale. These techniques can reveal how fatigue-induced changes in protein structure affect their mechanical behavior and interaction with other cellular components. For example, AFM can be used to measure the stiffness of individual muscle fibers or the force generated by single actin-myosin interactions, providing insights into how fatigue impacts muscle function at the molecular level.221
Moreover, fatigue-induced oxidative stress and inflammation can lead to modifications of muscle proteins through processes such as oxidation, nitrosylation, and phosphorylation. These post-translational modifications can alter the function and stability of proteins involved in muscle contraction and signaling. Understanding these molecular changes can inform the development of interventions to protect against fatigue-induced muscle damage and enhance recovery.222
Conditioning programs that improve muscular endurance and strategies to manage fatigue during sports are crucial for mitigating these risks. Such programs typically include exercises designed to enhance aerobic capacity and muscular stamina, allowing athletes to maintain higher levels of performance for longer periods.223 Interval training, resistance training with high repetitions, and circuit training are effective methods for building muscular endurance. Additionally, incorporating plyometric and neuromuscular training can improve the muscles’ ability to generate force quickly and maintain stability under fatigue.224
Proper hydration, nutrition, and rest are also critical components of managing fatigue. Adequate fluid intake helps maintain electrolyte balance and muscle function, while proper nutrition provides the necessary substrates for energy production and recovery. Ensuring athletes get sufficient rest and recovery time between training sessions allows for the repair and adaptation of muscle tissues, reducing the cumulative effects of fatigue.225
Furthermore, real-time monitoring of fatigue using wearable technology can help coaches and athletes adjust training loads and intensities to prevent overtraining and reduce injury risk. Wearable devices that track heart rate, muscle activity, and movement patterns can provide valuable feedback on an athlete’s fatigue levels, enabling personalized training adjustments.226
Incorporating these biophysical and molecular biology insights into training and competition routines can significantly enhance an athlete’s resilience to fatigue, improve knee stability, and reduce the likelihood of ACL injuries. By addressing both the physiological and molecular aspects of fatigue, sports professionals can develop comprehensive approaches to safeguard athletes’ knee health and optimize their performance.
Molecular Biophysics and Physiological Factors
Molecular biophysics provides a deeper understanding of how these physiological factors influence the mechanical properties of the ACL at the cellular and molecular levels. Hormonal influences, for example, affect the gene expression of collagen-producing cells (fibroblasts) in the ACL.227 Estrogen receptors on fibroblasts can modulate the synthesis and degradation of collagen, altering the ligament’s structural integrity. High estrogen levels can lead to decreased collagen cross-linking, resulting in a more compliant and less robust ACL. This hormonal modulation involves changes in the activity of enzymes such as lysyl oxidase, which is crucial for forming stable collagen cross-links. Reduced activity of these enzymes under the influence of estrogen can weaken the collagen network, making the ACL more prone to injury under mechanical stress.228
Additionally, hormonal variations influence the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). MMPs are enzymes that degrade extracellular matrix components, including collagen, while TIMPs regulate MMP activity. Elevated estrogen levels can increase MMP expression, leading to enhanced collagen breakdown and further compromising ACL integrity. Understanding these molecular interactions helps in developing targeted interventions to maintain ligament strength during hormonal fluctuations.229
Muscle strength and neuromuscular control are linked to molecular pathways that regulate muscle growth, adaptation, and coordination. The interaction between mechanical loading and cellular signaling pathways, such as the Akt/mTOR pathway, influences muscle hypertrophy and strength. This pathway regulates protein synthesis and muscle growth by activating key transcription factors and ribosomal proteins involved in muscle fiber production.230 Effective neuromuscular control relies on the precise regulation of ion channels and neurotransmitter release at the neuromuscular junction, which are modulated by molecular mechanisms. Calcium ions play a pivotal role in this process, as their influx into the presynaptic terminal triggers the release of acetylcholine, which then binds to receptors on the muscle cell membrane to initiate contraction. The regulation of calcium ion flow and neurotransmitter release is tightly controlled by voltage-gated ion channels and various signaling molecules, ensuring rapid and coordinated muscle responses.231
In addition, the RhoA/ROCK pathway plays a significant role in the regulation of cytoskeletal dynamics and muscle contraction. Activation of this pathway influences actin-myosin interactions and the organization of the cytoskeleton, which are essential for maintaining muscle tension and force generation. Understanding how mechanical stress activates these molecular pathways can inform training regimens that optimize muscle function and coordination.232
Fatigue impacts the biochemical environment within muscles, including the accumulation of metabolites such as lactate and hydrogen ions, which can impair muscle contraction and performance. At the molecular level, fatigue affects calcium handling within muscle fibers, disrupting excitation-contraction coupling and reducing force production. The sarcoplasmic reticulum (SR) plays a crucial role in storing and releasing calcium ions during muscle contraction.233 During fatigue, the efficiency of calcium reuptake by the SR is diminished due to the reduced activity of the calcium ATPase pump. This leads to prolonged calcium presence in the cytosol, which can impair the relaxation phase of muscle contraction and lead to sustained low-force contractions.234
Moreover, fatigue-induced oxidative stress results in the generation of reactive oxygen species (ROS), which can damage cellular components, including proteins, lipids, and DNA. ROS can modify contractile proteins such as actin and myosin, reducing their functionality and contributing to decreased muscle performance.235 Antioxidant defense mechanisms, including the activation of nuclear factor erythroid 2–related factor 2 (Nrf2) pathways, play a critical role in mitigating oxidative damage and preserving muscle function under fatigue conditions.236
Understanding these molecular processes can inform strategies to optimize training and recovery protocols, enhancing muscle performance and reducing injury risk. For instance, interventions that target the Akt/mTOR pathway can promote muscle hypertrophy and strength gains, while strategies that improve calcium handling and ion channel function can enhance neuromuscular control.237 Nutritional interventions, such as supplementation with antioxidants, can mitigate the effects of oxidative stress and preserve muscle function during prolonged exercise. Additionally, the use of specific training regimens designed to optimize the balance between high-intensity and recovery phases can enhance muscle endurance and delay the onset of fatigue.238
Furthermore, molecular biophysics provides insights into the repair and regeneration of injured ACL tissue. Growth factors such as TGF-beta and IGF-1 play significant roles in promoting fibroblast proliferation and collagen synthesis, essential for the healing process. Understanding the molecular signals that drive tissue repair can lead to the development of targeted therapies that enhance the natural healing processes or provide bioengineered solutions to strengthen the ACL post-injury.239
Molecular biophysics also sheds light on the biomechanics of ligamentous tissues at a nanoscale level. Techniques such as atomic force microscopy (AFM) and optical tweezers allow researchers to measure the mechanical properties of collagen fibers and other extracellular matrix components with high precision. These tools can reveal how molecular interactions and structural changes at the nanoscale contribute to the overall mechanical strength and resilience of the ACL.240
For example, AFM can be used to measure the stiffness and elasticity of individual collagen fibrils, providing insights into how hormonal fluctuations, mechanical loading, and fatigue influence these properties. This information can inform the design of biomaterials for ACL reconstruction that mimic the natural mechanical properties of the ligament, enhancing the success of surgical interventions.241
Incorporating these biophysical and molecular biology insights into training and rehabilitation programs can lead to more effective strategies for enhancing athletic performance, reducing injury incidence, and improving recovery outcomes.242 By understanding the underlying molecular and cellular mechanisms, trainers and therapists can design exercises that specifically target the critical aspects of neuromuscular function. This holistic approach can improve athletic performance, reduce injury incidence, and contribute to more effective rehabilitation protocols for those recovering from ACL injuries.243
By leveraging advanced molecular and biophysical techniques, we can develop more sophisticated and personalized approaches to sports medicine, ensuring that athletes achieve optimal performance while minimizing the risk of injury. This integrated understanding underscores the importance of considering both macroscopic and microscopic factors in managing ACL health and developing comprehensive strategies for injury prevention and rehabilitation (Table 5).
1. Genetic Factors
Genetic predispositions also play a significant role in determining an individual’s susceptibility to ACL injuries. Variations in genes related to collagen production, such as COL1A1 and COL5A1, can affect the structural integrity of the ACL. These genes encode types I and V collagen, respectively, which are critical components of ligament tissue.244 Polymorphisms in these genes may lead to alterations in collagen synthesis, cross-linking, and fibril formation, resulting in weaker collagen fibers and an increased risk of ligament injuries. For instance, certain polymorphisms in COL1A1 are associated with a reduced ability to produce strong, well-organized collagen fibers, making the ligament less capable of withstanding mechanical stress.245
Additionally, genes involved in muscle strength, neuromuscular coordination, and joint stability can influence an individual’s overall injury risk profile. Variations in genes such as ACTN3, which encodes alpha-actinin-3, a protein critical for fast-twitch muscle fibers, can affect muscle performance and fatigue resistance.246 Individuals with certain polymorphisms in ACTN3 may have reduced fast-twitch muscle function, impacting their ability to generate rapid and powerful muscle contractions needed to stabilize the knee during dynamic activities.247
Moreover, polymorphisms in genes involved in neuromuscular function, such as those encoding for neurotrophic factors (e.g., BDNF), ion channels, and receptors involved in neurotransmitter release and uptake, can affect neuromuscular coordination and proprioception. These genetic variations can lead to differences in the efficiency of neural signaling pathways that control muscle activation and coordination, potentially increasing the risk of improper joint mechanics and subsequent ACL injury.248
Genes involved in the inflammatory response and tissue repair also play a role in injury susceptibility and recovery. Variations in genes such as IL-6, which encodes interleukin-6, a cytokine involved in inflammation and immune response, can influence the extent and duration of inflammation following an injury. Individuals with certain IL-6 polymorphisms may experience prolonged or excessive inflammation, potentially hindering the healing process and increasing the risk of re-injury.249
Understanding these genetic predispositions through genetic testing can provide valuable insights into an individual’s risk factors for ACL injuries. Personalized training programs based on genetic profiles could potentially be used to identify high-risk individuals and tailor prevention strategies accordingly. For example, individuals with genetic markers associated with weaker collagen or reduced muscle strength may benefit from targeted strength training and neuromuscular conditioning exercises designed to enhance joint stability and improve muscle performance.250
Additionally, genetic information can inform nutritional and supplementation strategies to support tissue health and recovery. For instance, individuals with variations in genes related to collagen synthesis may benefit from supplements that support collagen production, such as vitamin C, lysine, and proline. Similarly, those with genetic predispositions to increased inflammation may benefit from anti-inflammatory diets rich in omega-3 fatty acids and antioxidants.251
From a molecular biophysics perspective, the structural and mechanical properties of the ACL are closely linked to the molecular composition and organization of its collagen fibers. Collagen fibrils are composed of triple-helical molecules that form a highly ordered, hierarchical structure. The strength and stiffness of these fibrils are influenced by the degree of cross-linking between collagen molecules, which is regulated by enzymes such as lysyl oxidase. Genetic variations that affect the expression or activity of these enzymes can lead to differences in the mechanical properties of the ACL, making it more or less susceptible to injury.252
The mechanical properties of the ACL are also influenced by the composition and organization of the extracellular matrix (ECM), which provides structural support to the ligament. The ECM is composed of a complex network of proteins, glycoproteins, and proteoglycans that interact with collagen fibers to modulate their mechanical behawior.253 Genetic variations that affect the synthesis or degradation of ECM components can alter the mechanical properties of the ACL, influencing its ability to withstand mechanical stress.
Advanced imaging techniques, such as atomic force microscopy (AFM) and electron microscopy, allow researchers to visualize and measure the mechanical properties of collagen fibers and ECM components at the nanoscale. These techniques provide insights into how genetic variations affect the structure and mechanical behavior of the ACL, informing the development of targeted interventions to strengthen the ligament and reduce the risk of injury.254
Furthermore, the interaction between mechanical loading and cellular signaling pathways, such as the mechanotransduction pathways, plays a crucial role in regulating the adaptation of the ACL to mechanical stress. Mechanotransduction involves the conversion of mechanical signals into biochemical signals that regulate cellular responses, such as gene expression and protein synthesis. Integrins, focal adhesion complexes, and other mechanosensitive proteins mediate this process by transmitting mechanical signals from the ECM to the cell interior. Genetic variations that affect the expression or function of these proteins can influence the cellular response to mechanical loading, affecting the adaptation of the ACL to mechanical stress.255
For example, the integrin-mediated signaling pathways regulate the expression of genes involved in collagen synthesis and degradation, influencing the mechanical properties of the ACL. The activation of these pathways can also induce the production of growth factors, such as TGF-beta and IGF-1, which promote fibroblast proliferation and collagen synthesis, enhancing the strength and repair capacity of the ligament.256
Understanding the molecular mechanisms underlying the adaptation of the ACL to mechanical stress can inform the development of personalized training and rehabilitation programs that optimize the mechanical loading conditions to enhance ligament strength and reduce the risk of injury. For instance, exercises that apply controlled mechanical loading to the ACL can stimulate the activation of mechanotransduction pathways, promoting the synthesis of collagen and other ECM components to strengthen the ligament.257
The integration of genetic testing with advanced molecular and biophysical techniques can further refine personalized prevention and rehabilitation strategies. For example, biomechanical assessments using motion capture technology and force plate analysis can provide detailed insights into an individual’s movement patterns and joint mechanics. Combining this data with genetic information can help identify specific biomechanical deficits and develop customized training programs to address these issues.258
Moreover, understanding the molecular pathways influenced by genetic variations can lead to the development of targeted therapies and interventions. For example, research into the molecular mechanisms underlying the effects of specific genetic polymorphisms on collagen synthesis and degradation can inform the development of pharmacological agents or gene therapies to enhance ligament strength and repair.259
Incorporating genetic testing and personalized training programs into sports medicine practices can significantly improve the effectiveness of injury prevention and rehabilitation efforts. By leveraging genetic insights, trainers and healthcare professionals can design more precise and individualized interventions, reducing the risk of ACL injuries and optimizing athletic performance and recovery outcomes. This comprehensive approach underscores the importance of considering genetic, molecular, and biomechanical factors in developing strategies to protect and enhance the health of the ACL and overall joint function.
2. Recovery and Rehabilitation
Understanding the physiological factors influencing ACL injuries is crucial for developing effective recovery and rehabilitation protocols. Hormonal influences, for example, can significantly affect the healing process. Estrogen has been shown to impact collagen synthesis and wound healing, suggesting that hormonal regulation may play a role in the rehabilitation of ACL injuries.260 Estrogen can influence the proliferation and migration of fibroblasts, which are essential for collagen production and tissue repair. Elevated levels of estrogen might enhance the initial healing phase by promoting cellular activities that regenerate ligament tissues, but they might also alter collagen cross-linking, potentially affecting the long-term strength of the repaired ACL.261
Personalized rehabilitation programs that consider hormonal fluctuations, muscle strength imbalances, and neuromuscular control deficits can optimize recovery and reduce the risk of re-injury.262 For instance, during phases of the menstrual cycle when estrogen levels are high, specific rehabilitation strategies might be employed to harness the beneficial effects of estrogen on cell proliferation while also mitigating potential risks related to ligament laxity. This approach could involve timing certain physical therapy exercises or modalities to align with hormonal cycles, thereby maximizing the body’s natural repair processes while minimizing vulnerabilities.263
Muscle strength imbalances, such as those between the quadriceps and hamstrings, are also critical to address in ACL rehabilitation. The quadriceps are often stronger than the hamstrings, which can create an imbalance that increases the strain on the ACL during activities. Targeted strengthening exercises for the hamstrings, as well as the hip and core muscles, can improve overall knee stability and reduce the likelihood of compensatory movements that might lead to re-injury.264 Isokinetic testing can be used to identify specific imbalances and tailor strength training protocols accordingly, ensuring a balanced and supportive musculature around the knee.265
Neuromuscular control deficits are another vital component to consider in personalized rehabilitation programs. Effective neuromuscular control ensures that the muscles surrounding the knee joint respond appropriately to dynamic loads and maintain joint stability. Rehabilitation protocols can include neuromuscular training exercises that focus on enhancing proprioception, balance, and coordination. Techniques such as balance board exercises, agility drills, and plyometric training can improve the reflexive stabilization of the knee, which is crucial for preventing re-injury during high-intensity or unpredictable movements.266
From a molecular biophysics perspective, understanding the interplay between mechanical forces and cellular responses during rehabilitation can provide insights into optimizing tissue healing and strengthening. The application of mechanical loading through physical therapy exercises can stimulate mechanotransduction pathways in fibroblasts, leading to the upregulation of collagen synthesis and the strengthening of the repaired ligament.267 Controlled mechanical loading can enhance the alignment and organization of collagen fibers, improving the biomechanical properties of the healing ACL. This approach involves carefully calibrated exercises that apply appropriate stress to the ligament, encouraging adaptive remodeling without causing further damage.268
Additionally, molecular biophysics explores the role of integrins and focal adhesions in mechanotransduction. Integrins are transmembrane receptors that connect the extracellular matrix to the cytoskeleton, facilitating the transmission of mechanical signals into biochemical responses. Focal adhesions are complex assemblies of proteins that link integrins to the actin cytoskeleton and serve as signaling hubs.269 When mechanical stress is applied to the ligament, integrins cluster and recruit focal adhesion proteins, activating intracellular signaling pathways such as the MAPK/ERK and RhoA/ROCK pathways. These pathways regulate gene expression and protein synthesis, promoting tissue repair and adaptation. Understanding the molecular details of these processes can inform the design of rehabilitation protocols that optimize the mechanical loading conditions to enhance ligament healing and strength.270
Moreover, advancements in molecular biology have identified key growth factors and cytokines involved in tissue repair, such as transforming growth factor-beta (TGF-β) and insulin-like growth factor-1 (IGF-1). These molecules play crucial roles in modulating cellular activities during the healing proces.271 Therapeutic interventions that incorporate these growth factors, either through localized delivery systems or systemic administration, could accelerate ligament healing and enhance the structural integrity of the repaired ACL. Combining these molecular therapies with targeted physical rehabilitation could provide a synergistic effect, optimizing the overall recovery proces.272
Molecular biophysics also provides insights into the role of the extracellular matrix (ECM) in ligament healing. The ECM is a dynamic structure that provides mechanical support and regulates cellular behavior through biochemical and mechanical cues.273 During the healing process, the composition and organization of the ECM change, influencing the mechanical properties of the repaired ligament. Techniques such as atomic force microscopy (AFM) and rheometry can be used to measure the mechanical properties of the ECM at different stages of healing, providing insights into how rehabilitation exercises can be designed to modulate ECM remodeling and improve ligament strength.274
Incorporating genetic testing into personalized rehabilitation programs can further enhance their effectiveness. Genetic testing can identify individual variations in genes related to collagen production, inflammation, and muscle performance. For example, individuals with specific polymorphisms in the COL1A1 or IL-6 genes may require tailored rehabilitation strategies that account for their unique genetic predispositions.275 Understanding these genetic factors can help in designing personalized protocols that enhance the efficacy of rehabilitation and reduce the risk of adverse outcomes.276
The integration of wearable technology and biofeedback systems can further optimize personalized rehabilitation. Wearable sensors can monitor joint movement, muscle activity, and loading patterns in real-time, providing valuable data that can be used to continuously adjust rehabilitation exercises. Biofeedback systems can provide immediate feedback to patients, helping them improve their movement patterns and muscle activation during exercises.277 These technologies can ensure that rehabilitation exercises are performed correctly and effectively, enhancing the overall recovery process.
In conclusion, a comprehensive approach that integrates hormonal considerations, muscle strength balancing, neuromuscular training, molecular biology insights, and advanced technology can significantly enhance the rehabilitation of ACL injuries. By tailoring rehabilitation programs to the individual needs and physiological profiles of patients, healthcare professionals can optimize recovery, reduce the risk of re-injury, and ultimately improve long-term outcomes for individuals recovering from ACL injuries. Understanding the intricate molecular and biophysical mechanisms underlying ligament healing and adaptation can inform the development of more effective rehabilitation protocols, ensuring that athletes and patients achieve optimal recovery and return to their activities with reduced risk of future injuries.
3. Nutritional Influences
Nutrition also plays a vital role in maintaining the physiological health of the ACL and surrounding muscles. Adequate intake of proteins, vitamins (such as Vitamin C for collagen synthesis), and minerals (such as calcium and magnesium for muscle function) supports the structural integrity and functionality of the ACL. Proteins provide the essential amino acids required for the synthesis of new collagen fibers and the repair of muscle tissues.278 Vitamin C is a critical cofactor in the hydroxylation of proline and lysine residues in collagen, a process necessary for the stability and strength of collagen triple helices. Minerals like calcium and magnesium are essential for muscle contraction and relaxation, contributing to the overall balance and coordination needed for joint stability.279
Omega-3 fatty acids have anti-inflammatory properties that can aid in recovery post-injury. These fatty acids can reduce the production of pro-inflammatory cytokines and eicosanoids, thereby mitigating inflammation and promoting a more favorable environment for tissue repair.280 Additionally, omega-3 fatty acids can enhance the resolution phase of inflammation, aiding in the transition from the inflammatory response to tissue regeneration and healing.281
Nutritional strategies that focus on providing the necessary building blocks for collagen synthesis and muscle repair can enhance ligament resilience and support overall joint health. For instance, incorporating a diet rich in antioxidants can protect against oxidative stress, which can degrade collagen and impair muscle function. Foods high in antioxidants, such as berries, leafy greens, and nuts, can help neutralize free radicals and support tissue repair processes.282
Furthermore, specific amino acids such as glycine, proline, and lysine are particularly important for collagen synthesis. Ensuring adequate intake of these amino acids through dietary sources or supplementation can promote optimal collagen production and enhance the structural integrity of the ACL.283 Collagen supplements, often derived from bovine or marine sources, can also provide these critical amino acids and have been shown to support joint health and recovery.
From a molecular biophysics perspective, nutrition directly impacts the biochemical and mechanical properties of the ACL and muscle tissues. The process of collagen synthesis involves a series of post-translational modifications that are dependent on the availability of specific nutrients. For example, the hydroxylation of proline and lysine residues in collagen by prolyl and lysyl hydroxylase enzymes requires Vitamin C as a cofactor.284 This hydroxylation is crucial for the formation of stable collagen triple helices, which confer tensile strength and resistance to mechanical stress. Deficiencies in Vitamin C can lead to impaired collagen synthesis and weakened ligament structures.285
Additionally, the cross-linking of collagen fibers, a process essential for the mechanical stability and durability of the ACL, is influenced by the availability of nutrients that support enzymatic activity. Lysyl oxidase, an enzyme that catalyzes the formation of covalent cross-links between collagen molecules, requires copper as a cofactor. Adequate dietary intake of copper is therefore essential for the optimal function of lysyl oxidase and the maintenance of strong collagen networks within the ACL.286
Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exert their anti-inflammatory effects by modulating the activity of nuclear factor-kappa B (NF-κB) and other transcription factors involved in the inflammatory response.287 These fatty acids can also influence the expression of genes involved in collagen synthesis and degradation, thereby supporting the maintenance and repair of ligament tissues. The incorporation of omega-3 fatty acids into cell membranes can also enhance membrane fluidity and cell signaling, further promoting tissue health and resilience.288
In summary, physiological factors including hormonal levels, muscle strength, neuromuscular control, and fatigue significantly influence the risk of ACL injuries. Hormonal fluctuations can alter collagen synthesis and ligament laxity, while muscle imbalances and poor neuromuscular control can compromise joint stability. Fatigue exacerbates these issues by impairing muscle function and coordination.
Molecular biophysics provides valuable insights into the underlying mechanisms by which these factors affect the mechanical properties and structural integrity of the ACL. Understanding the molecular dynamics of collagen fiber formation, the mechanotransduction pathways that regulate cellular responses to mechanical stress, and the genetic factors that predispose individuals to weaker ligament structures can inform more effective prevention and rehabilitation strategies.289
By understanding the interplay between physiological factors, genetic predispositions, and molecular mechanisms, researchers and clinicians can develop targeted prevention, training, and rehabilitation strategies to reduce the risk of ACL injuries and enhance athlete performance. Integrating these insights into personalized approaches can optimize injury prevention and recovery, ultimately improving the health and safety of individuals engaged in physical activities.290 Personalized nutrition plans, tailored exercise regimens, and advanced therapeutic interventions that consider an individual’s unique physiological and genetic profile can significantly reduce the incidence of ACL injuries and improve rehabilitation outcomes.
Moreover, leveraging technology such as wearable sensors and biofeedback devices can provide real-time data on an individual’s biomechanical performance and physiological status, enabling continuous optimization of training and rehabilitation programs.291 This comprehensive and integrated approach, combining molecular biophysics, nutrition, personalized medicine, and advanced technology, represents the future of sports medicine and injury prevention, ensuring that athletes and active individuals can maintain peak performance while minimizing the risk of injury.
Molecular biophysics further elucidates the complex interactions between nutrition, biomechanics, and cellular function. For example, the mechanical loading of the ACL during physical activity induces cellular signaling pathways that regulate gene expression and protein synthesis.292 The interaction between mechanical forces and cellular mechanotransduction mechanisms, such as the integrin-mediated activation of focal adhesion kinase (FAK) and the subsequent activation of the MAPK/ERK pathway, plays a crucial role in ligament adaptation and repair. Nutritional status can modulate these signaling pathways by influencing the availability of cofactors and substrates required for enzyme activity and protein synthesis.293
Incorporating molecular and biophysical insights into sports nutrition and rehabilitation not only enhances our understanding of ligament health but also paves the way for innovative interventions that can prevent injuries and accelerate recovery. For instance, the development of nutraceuticals and functional foods designed to support collagen synthesis, reduce inflammation, and enhance muscle performance can provide athletes with targeted nutritional support that aligns with their physiological needs.294
By integrating these advanced scientific insights into practical applications, sports medicine professionals can offer more effective, evidence-based interventions that promote long-term joint health and athletic performance. This holistic approach underscores the importance of considering the intricate molecular and biophysical factors that contribute to ligament health and resilience, ultimately leading to improved outcomes for athletes and active individuals.
Discussion
The interaction of biomechanical, anatomical, and physiological factors creates a complex landscape in which ACL injuries occur. Understanding these interactions is essential for developing comprehensive injury prevention programs. Each factor contributes uniquely to the risk profile, and their interplay can exacerbate or mitigate injury risk (Figure 2).
Biomechanical studies emphasize the importance of proper technique and strength training to mitigate risky movements. For instance, incorrect landing mechanics, such as excessive knee valgus or improper hip alignment, significantly increase ACL strain. Strengthening programs targeting the quadriceps, hamstrings, and hip muscles can correct these mechanics, reducing the risk of injury. Plyometric and agility drills designed to enhance proprioception and neuromuscular control are critical components of these programs. By improving the body’s ability to react to dynamic loads and maintain proper joint alignment, these drills help athletes avoid the movement patterns most associated with ACL injuries.295
Anatomical considerations suggest the potential for screening and personalized interventions for individuals at higher risk. Anatomical variations, such as a narrow intercondylar notch or increased tibial slope, have been linked to a higher incidence of ACL injuries. Screening for these risk factors using imaging techniques like MRI can identify individuals who may benefit from targeted preventive measures. Personalized interventions might include customized training regimens that focus on specific weaknesses or imbalances identified during screening. For example, individuals with a narrow intercondylar notch might need to avoid certain high-risk activities or receive specialized training to reinforce their ligament stability.296
Physiological insights highlight the need for targeted neuromuscular training and possibly hormonal considerations in injury prevention strategies. Neuromuscular training aims to enhance the coordination and timing of muscle activations around the knee joint, which is crucial for maintaining joint stability during dynamic movements. Exercises that improve balance, proprioception, and reaction time can significantly reduce the likelihood of improper knee loading and subsequent ACL injury. Furthermore, understanding hormonal influences on ligament properties can inform the timing and type of preventive measures.297 For instance, women may be more susceptible to ACL injuries during certain phases of their menstrual cycle when estrogen levels peak and ligament laxity increases. Hormonal monitoring and interventions, such as scheduling high-risk training activities during phases of lower estrogen levels, could be considered to mitigate this risk.298
Molecular biophysics further deepens our understanding of these interactions by elucidating the cellular and molecular mechanisms that underlie tissue response to mechanical stress. The role of mechanotransduction pathways in fibroblasts, which respond to mechanical loading by adjusting collagen synthesis and degradation, is crucial for ligament health. Proper mechanical loading through strength training can stimulate these pathways, promoting the maintenance and repair of the ACL. Conversely, excessive or improper loading can disrupt these pathways, leading to tissue damage and increased injury risk.299
Additionally, molecular studies on genetic predispositions offer insights into personalized preventive strategies. Variations in genes related to collagen production and neuromuscular coordination can affect an individual’s susceptibility to ACL injuries. Genetic testing can identify these variations, allowing for personalized training programs that address specific vulnerabilities. For instance, individuals with genetic markers indicating weaker collagen may benefit from enhanced strength training and nutritional support to bolster ligament resilience.300
Integrating these diverse insights into a cohesive injury prevention strategy requires a multidisciplinary approach. Collaboration among sports scientists, orthopedic surgeons, physical therapists, and genetic counselors can lead to the development of comprehensive programs that address the multifaceted nature of ACL injury risk. By combining biomechanical training, anatomical screening, physiological conditioning, and molecular insights, these programs can more effectively reduce the incidence of ACL injuries and enhance athlete performance and safety.301
In conclusion, the intricate interplay of biomechanical, anatomical, and physiological factors necessitates a holistic approach to ACL injury prevention. By leveraging advanced research and technology, we can develop more effective, personalized strategies that not only prevent injuries but also optimize overall athletic performance. This integrative approach represents the future of sports medicine, offering tailored solutions that address the unique needs of each individual athlete.
Integrated Approaches
Integrated approaches to ACL injury prevention and rehabilitation combine insights from biomechanics, anatomy, physiology, molecular biology, and personalized medicine to create comprehensive and effective strategies. By addressing the multifaceted nature of ACL injuries, these approaches aim to reduce injury incidence, optimize recovery, and enhance overall athletic performance.302
Biomechanically, integrated approaches emphasize the importance of movement analysis and correction. High-speed cameras and motion capture systems can identify faulty movement patterns that increase ACL strain, such as knee valgus or improper hip rotation during jumps and landings. Corrective exercises and drills can then be designed to specifically target these issues, improving technique and reducing injury risk.303
Anatomically, screening for structural vulnerabilities plays a crucial role. Advanced imaging technologies, such as MRI and CT scans, can detect anatomical features like a narrow intercondylar notch or a steep tibial slope that predispose individuals to ACL injuries. Personalized interventions, such as tailored strength training programs or surgical options, can be developed to address these anatomical risk factors.304
Physiologically, neuromuscular training programs are tailored to enhance proprioception, balance, and muscle coordination. Techniques such as dynamic stability exercises, balance board training, and agility drills improve the neuromuscular control of the knee joint, helping to stabilize the knee during high-impact activities. Additionally, the timing and intensity of these exercises can be adjusted based on individual needs and hormonal fluctuations, particularly in female athletes.305
From a molecular biology perspective, understanding the role of genetics and molecular pathways in ACL health allows for more targeted interventions. Genetic testing can identify individuals with polymorphisms in genes related to collagen synthesis, muscle strength, and inflammatory responses. These individuals can benefit from customized training and nutritional plans that support optimal ligament health and repair. For example, those with genetic markers for weaker collagen may be advised to incorporate collagen-boosting supplements and foods rich in vitamin C, zinc, and copper into their diet.306
Moreover, molecular biophysics sheds light on the mechanotransduction processes that influence ligament adaptation to mechanical stress. The application of controlled mechanical loads through resistance training can activate signaling pathways in fibroblasts, promoting collagen synthesis and strengthening the ACL. Conversely, avoiding excessive or improper loading that could disrupt these pathways is crucial. Understanding these processes can help in designing exercise regimens that balance stress and recovery, fostering ligament resilience.307
Technological advancements, such as wearable sensors and biofeedback devices, play a pivotal role in integrated approaches. Wearable technology can monitor real-time biomechanical data, providing immediate feedback on movement patterns and muscle activity. This data can be used to adjust training loads and techniques on the fly, ensuring that athletes perform exercises correctly and safely. Biofeedback devices can also help athletes develop better awareness of their body movements, leading to more effective neuromuscular training.308
Furthermore, the integration of nutritional science into injury prevention and rehabilitation strategies ensures that athletes receive the necessary nutrients to support muscle and ligament health. Diet plans rich in anti-inflammatory foods, proteins, and micronutrients like vitamin D and omega-3 fatty acids can enhance recovery and reduce the risk of re-injury. Personalized nutrition counseling based on genetic and physiological profiles can further optimize these dietary interventions.309
In rehabilitation, combining physical therapy with molecular and genetic insights can accelerate recovery and improve outcomes. For example, therapies that promote the expression of growth factors and cytokines involved in tissue repair can be paired with targeted physical exercises to enhance ligament healing. Additionally, understanding an individual’s genetic predispositions can inform the selection of therapeutic modalities and the intensity of rehabilitation exercises.310
Integrated approaches also benefit from interdisciplinary collaboration among healthcare providers, including orthopedic surgeons, physical therapists, sports scientists, nutritionists, and genetic counselors. This collaborative effort ensures that all aspects of an athlete’s health are considered, from mechanical alignment and muscle strength to genetic predispositions and nutritional status. Such a comprehensive approach not only enhances the effectiveness of injury prevention and rehabilitation programs but also supports the overall well-being and performance of athletes.311
In conclusion, integrated approaches to ACL injury prevention and rehabilitation represent the future of sports medicine. By combining biomechanical analysis, anatomical screening, physiological conditioning, molecular biology, and personalized interventions, these approaches provide a holistic and effective strategy for reducing ACL injuries and optimizing athlete health and performance. This multidimensional approach ensures that each athlete receives tailored care that addresses their unique needs and risk factors, ultimately leading to better outcomes and a reduced risk of injury (Table 6).
1. Injury Prevention Programs
Comprehensive injury prevention programs, when augmented by molecular biophysics, can be significantly more effective in addressing ACL injury risk by targeting the underlying molecular and biophysical processes. Biomechanical training, which aims to correct movement patterns and reduce harmful stresses on the ACL, directly influences the mechanotransduction pathways in ligament fibroblasts. Molecular biophysics reveals that mechanical forces experienced during specific exercises activate intracellular signaling pathways, such as the integrin-FAK (focal adhesion kinase) pathway.312 This activation regulates the synthesis and organization of collagen fibers within the ACL, affecting its tensile strength and elasticity. By optimizing biomechanical training to modulate these pathways, we can enhance the structural integrity and resilience of the ACL.313
Strength conditioning not only builds muscle mass but also influences the molecular environment of the ACL. Resistance exercises induce mechanical strain on muscles and connective tissues, triggering biochemical responses in the muscle cells and surrounding ECM. The Akt/mTOR pathway, crucial for muscle hypertrophy and strength, also impacts the ECM by promoting the synthesis of matrix proteins and growth factors. Molecular biophysics studies how increased mechanical loading during strength training affects ECM remodeling and collagen turnover in the ACL, thus enhancing its ability to withstand dynamic forces.314 Understanding these interactions helps in designing strength programs that not only improve muscle function but also support ligament health through optimized ECM adaptation.315
Neuromuscular education improves proprioception, balance, and coordination, which are vital for maintaining joint stability and preventing ACL injuries. At a molecular level, neuromuscular training affects synaptic efficiency and neural plasticity.316 Exercises that enhance proprioception and coordination influence ion channel dynamics and neurotransmitter release at the neuromuscular junction. Molecular biophysics provides insights into how these changes improve synaptic transmission and muscle activation patterns, which are critical for timely and effective responses to dynamic movements. Enhanced neuromuscular control helps in maintaining proper joint alignment and reducing stress on the ACL, which is crucial for injury prevention.317
Nutritional strategies, guided by molecular biophysics, are essential for supporting the physiological health of the ACL and surrounding tissues. For instance, adequate intake of amino acids and vitamins directly impacts collagen biosynthesis.318 Molecular biophysics elucidates how specific nutrients influence the post-translational modification of collagen molecules, such as hydroxylation of proline and lysine residues, which is necessary for stable collagen fibril formation. Omega-3 fatty acids, known for their anti-inflammatory properties, affect cellular signaling pathways involved in inflammation and tissue repair. By modulating these pathways, omega-3s can reduce inflammatory responses around the ACL and promote a more favorable environment for ligament recovery and strengthening.319
Advanced technologies, such as motion capture systems and wearable sensors, provide real-time data on movement mechanics and biomechanical stresses. Molecular biophysics can interpret how these technologies affect the mechanical and biochemical environment of the ACL.320 For example, real-time feedback on joint angles and forces helps in adjusting training techniques to minimize excessive stress on the ACL. Molecular insights into how mechanical stress influences collagen remodeling and cellular responses enable more precise adjustments to training protocols, enhancing the efficacy of injury prevention programs.321
Personalized injury prevention strategies benefit greatly from molecular biophysics by allowing for tailored interventions based on individual genetic and molecular profiles. Genetic variations affecting collagen production or muscle strength can be identified through genetic testing, and molecular biophysics provides insights into how these variations influence ligament and muscle properties.322 By understanding these molecular mechanisms, personalized training and rehabilitation programs can be designed to address specific genetic predispositions and optimize injury prevention efforts.
In summary, integrating molecular biophysics into injury prevention programs enhances our understanding of how biomechanical, strength, and neuromuscular interventions impact the ACL at the molecular and cellular levels. By elucidating the mechanisms of mechanotransduction, collagen remodeling, and cellular responses to training, we can develop more targeted and effective prevention strategies. This comprehensive approach not only improves ligament and muscle health but also optimizes overall injury prevention, leading to better outcomes for athletes and reduced risk of ACL injuries.
2. Screening and Risk Assessment
Implementing comprehensive screening programs to identify individuals at high risk of anterior cruciate ligament (ACL) injuries is essential for tailoring effective prevention strategies. These programs utilize a blend of anatomical, physiological, and biochemical factors to generate a detailed risk profile. Key tools such as the Landing Error Scoring System (LESS) and functional movement screenings play a vital role in this proces.323 The LESS assesses landing techniques by identifying potentially harmful movement patterns, such as excessive knee valgus or improper alignment, which are linked to increased ACL strain. Functional movement screenings evaluate a variety of movements and postures to uncover abnormalities in joint stability and alignment that could predispose an individual to injury.324
Incorporating molecular biophysics into these screening programs enhances the accuracy of risk assessments by addressing the underlying molecular mechanisms involved in ACL injuries. One significant advancement is the use of genetic screening to identify variations in genes associated with collagen synthesis and structure.325 For example, variations in the COL1A1 and COL5A1 genes, which encode for type I and type V collagen respectively, can influence ligament strength and elasticity. These genetic markers can indicate a predisposition to weaker or more elastic ligaments, which may be more susceptible to injury.326 By understanding these genetic predispositions, practitioners can tailor prevention strategies that address the specific needs of individuals based on their genetic profile.
Furthermore, biomechanical analyses, such as those involving motion capture technology and force platforms, provide insights into how mechanical loads affect the ACL at the molecular level. These analyses can reveal how different movement patterns impact the distribution of forces across the ACL and influence collagen remodeling.327 For instance, excessive or improper mechanical loading can disrupt collagen fiber alignment and lead to maladaptive cellular responses, increasing the risk of injury. Understanding these interactions at a molecular level allows for the development of targeted interventions that can modify movement patterns to reduce stress on the ACL.328
Molecular biophysics also extends to evaluating the biochemical environment surrounding the ACL. For example, research into the role of matrix metalloproteinases (MMPs), enzymes that degrade extracellular matrix components, provides insights into how excessive MMP activity might contribute to ligament degradation and injury risk.329 By assessing MMP levels and other biomarkers in individuals, practitioners can gain a better understanding of the biochemical factors influencing ACL health.330
Additionally, functional assessments that include molecular insights into muscle fatigue and strength can be invaluable. For example, understanding how fatigue impacts neuromuscular control and affects muscle activation patterns can help identify individuals with compromised stability or alignment.331 By integrating these molecular insights with functional assessments, practitioners can detect early signs of fatigue-related changes that might increase the risk of ACL injury.332
Hormonal assessments also offer important information by evaluating the effects of hormonal fluctuations on ligament properties. Tracking levels of hormones such as estrogen and relaxin, which influence ligament laxity and strength, can provide insights into how these hormonal changes may affect ACL vulnerability. This is particularly relevant for female athletes, as variations in these hormones throughout the menstrual cycle can impact ligamentous structures.333
Incorporating these molecular biophysics perspectives into screening programs enables a more comprehensive approach to ACL injury prevention. By integrating genetic, biochemical, and biomechanical data, practitioners can develop more personalized and effective prevention strategies. This holistic approach addresses both the structural and biochemical factors that contribute to ACL injuries, ultimately improving the efficacy of prevention programs and reducing the incidence of these common and debilitating injuries.
3. Rehabilitation Strategies
Integrating molecular biophysics into post-injury rehabilitation for ACL injuries provides a detailed understanding of the molecular and cellular processes underlying recovery, allowing for the refinement of rehabilitation strategies to improve outcomes. After an ACL injury, the primary cellular players in ligament repair are fibroblasts, which are responsible for producing collagen, particularly type I collagen, which is crucial for rebuilding the damaged ligament.334 Mechanical loading from rehabilitation exercises stimulates these fibroblasts via mechanotransduction pathways, which translate mechanical stress into biochemical signals that drive collagen synthesis and remodeling. Molecular biophysics explores how different mechanical loads affect collagen fiber alignment and cross-linking. Proper alignment and cross-linking of collagen fibers are essential for the ligament’s tensile strength and flexibility. Studies in this field examine how mechanical stress impacts these processes, helping to tailor rehabilitation exercises to optimize ligament repair and function.335
Transforming growth factor beta (TGF-β) is another critical player in ACL injury rehabilitation. TGF-β regulates the production and remodeling of the extracellular matrix (ECM), which includes collagen and other structural proteins.336 Progressive loading and exercise influence TGF-β signaling pathways, affecting ECM composition and the repair process. Molecular biophysics investigates how mechanical stress modulates TGF-β activity and its downstream effects on collagen deposition and ECM remodeling.337 By understanding these interactions, rehabilitation protocols can be adjusted to optimize TGF-β signaling, enhancing collagen production and improving tissue repair.
Neuromuscular exercises play a significant role in ACL rehabilitation by promoting neuroplasticity, which involves the formation of new neural connections and the strengthening of existing ones. This process relies on signaling pathways such as brain-derived neurotrophic factor (BDNF) and NMDA receptor-mediated synaptic plasticity.338 Molecular biophysics provides insights into how different types of neuromuscular training affect these pathways, which can improve motor control and joint stability. Strength training, in particular, affects motor unit recruitment and synchronization, which are crucial for effective muscle contraction and joint stabilization.339 Molecular biophysics studies how training-induced changes in ion channel function and intracellular calcium dynamics contribute to improved neuromuscular control, offering insights into how to design effective training programs for enhanced recovery.
Proprioception, or the sense of body position and movement, is also crucial in ACL rehabilitation. Proprioceptors detect changes in joint position and movement, and rehabilitation exercises that challenge sensory-motor integration can enhance proprioceptive function.340 Molecular biophysics explores the activation of mechanosensitive ion channels, such as Piezo1 and TRPV4, during proprioceptive exercises. These channels play a role in detecting mechanical stimuli and affecting sensory feedback and motor responses. Additionally, proprioceptive training can modulate neurotransmitter systems involved in sensory-motor integration.340 Increased levels of neurotransmitters such as serotonin and dopamine can enhance proprioceptive acuity and motor coordination. Biophysical studies of neurotransmitter release and receptor activity provide insights into how proprioceptive training affects sensory-motor pathways and helps in designing exercises that improve proprioceptive function and overall joint stability.
Progressive loading is a fundamental aspect of rehabilitation, involving the gradual increase of mechanical stress to stimulate cellular responses and promote tissue adaptation. Molecular biophysics investigates how mechanical stress activates mechanotransduction pathways, such as the mTOR signaling pathway, which regulates muscle protein synthesis and cell growth.341 By understanding how mechanical stress impacts cellular structures and signaling pathways, rehabilitation protocols can be optimized to maximize the benefits of progressive loading. Tendon adaptation to progressive loading includes changes in collagen composition, cross-linking, and mechanical properties.342 Molecular biophysics studies how varying loads influence these factors, providing insights into how to design loading protocols that enhance tendon strength and function while supporting recovery.
Functional exercises are designed to improve muscle strength and coordination by influencing gene expression related to muscle growth and repair. These exercises can upregulate myogenic regulatory factors and growth factors that are critical for muscle adaptation.343 Functional exercises also affect joint and ligament loading. Biophysical studies examine how dynamic movements impact joint mechanics and ligament stress, helping to design exercises that improve functional performance while minimizing the risk of re-injury.344 Understanding these effects allows for the development of exercises that enhance strength, coordination, and overall functional ability.
Monitoring biochemical markers related to inflammation, tissue repair, and muscle function provides valuable feedback on rehabilitation progress. Molecular biophysics helps interpret how these markers reflect cellular and molecular changes during rehabilitation, guiding adjustments to treatment strategies. Advanced imaging techniques, such as MRI and ultrasound, offer insights into tissue healing and adaptation at the molecular level.345 These techniques can assess changes in tissue composition, collagen alignment, and muscle function, providing detailed information to guide rehabilitation adjustments. By integrating molecular biophysics into ACL injury rehabilitation, practitioners gain a comprehensive understanding of how various strategies impact recovery at the cellular and molecular levels. This approach allows for the optimization of rehabilitation protocols to enhance recovery, prevent re-injury, and support a successful return to pre-injury activities.346 Ultimately, integrating molecular biophysics into rehabilitation not only improves our understanding of recovery processes but also enables the development of more effective, personalized treatment strategies that address individual needs and conditions.347
Conclusion
This review underscores the intricate and multifaceted nature of ACL injuries, which arise from a complex interplay of biomechanical forces, anatomical predispositions, and physiological conditions. Biomechanical forces are a primary factor, encompassing the various stresses and strains exerted on the knee joint during different activities. These forces can vary greatly depending on the type of movement, the intensity of physical activity, and the mechanical properties of the knee joint and surrounding structures. For instance, sudden changes in direction, excessive torsional forces, or high-impact activities can place significant strain on the ACL, potentially leading to injury. Movement patterns and joint alignment also play a critical role; improper technique or alignment during dynamic activities can exacerbate the risk of injury.
Anatomical predispositions further complicate the risk of ACL injuries. Variations in joint geometry, such as differences in femoral and tibial alignment, as well as the size and shape of the ACL itself, can influence susceptibility to injury. Additionally, anatomical features such as the Q-angle, which measures the alignment of the knee in relation to the hip and ankle, can impact the distribution of forces across the knee joint. Variations in ligament structure, including differences in ligament length, strength, and attachment points, also contribute to individual risk profiles. Understanding these anatomical variations is crucial for identifying individuals who may be more predisposed to ACL injuries.
Physiological conditions also play a significant role in the risk of ACL injuries. Factors such as muscle strength, flexibility, and neuromuscular control are essential for maintaining joint stability and absorbing mechanical forces. Weakness in the muscles that support the knee, particularly the quadriceps and hamstrings, can lead to reduced joint stability and increased risk of injury. Similarly, poor flexibility or imbalances in muscle strength can affect the ability to effectively absorb and distribute forces during dynamic movements. Neuromuscular control, which involves the coordination of muscle activity and joint position sense, is also critical. Impaired proprioception or delayed reaction times can increase the likelihood of ACL injuries by reducing an individual’s ability to respond to sudden changes or excessive stresses.
Future research should therefore focus on integrated approaches that combine these diverse factors to develop more effective prevention and rehabilitation strategies. This could involve a holistic examination of how biomechanical, anatomical, and physiological factors interact to influence ACL injury risk. For example, research could explore how specific movement patterns in combination with anatomical variations affect injury susceptibility, or how muscle strength and flexibility interact with joint alignment to impact injury risk.
An integrated approach would involve creating comprehensive models that consider all relevant factors, leading to more precise identification of high-risk individuals. Such models could inform the development of targeted prevention programs that address specific vulnerabilities identified through personalized assessments. This could include tailored strength and conditioning programs, individualized flexibility and neuromuscular training, and biomechanical assessments to correct movement patterns or alignment issues. Personalized approaches, grounded in a thorough understanding of individual risk profiles, may offer the most promising avenue for reducing the incidence and severity of ACL injuries. By focusing on these personalized strategies, we can enhance the effectiveness of both prevention and rehabilitation efforts, leading to improved outcomes and reduced recurrence rates. This comprehensive perspective not only deepens our understanding of ACL injuries but also fosters the development of evidence-based, individualized interventions that address the unique needs of each individual.