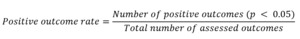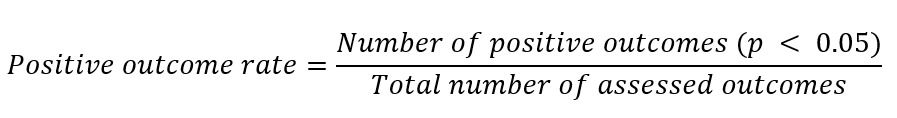Introduction
In primary care, the three most common musculoskeletal pain presentations are lower back, knee, and shoulder.1 Systematic reviews estimate the prevalence of low back pain at 23,2%,2 knee pain at 22.7%,3 and shoulder pain at 14.5%.4 These presentations are often associated with various underlying, especially chronic, conditions. Osteoarthritis (OA) is characterized by the progressive degeneration of articular cartilage, inflicting pain and functional disability. It is estimated that around 250 million people worldwide suffer from OA.5 Osteoporosis, the most frequent bone disorder globally, results from an imbalanced physiological processes of bone formation and bone reabsorption.6
The musculoskeletal system encompasses the bones, tendons, ligaments, muscles, and cartilage, all of which contain an extracellular matrix rich in collagen. Collagen is a structural protein that confers elasticity and transmits contractile force between fibers.7,8 Musculoskeletal disorders can be triggered by different factors, such as age, weight, hormonal levels, frequency, and physical activity levels. Populations susceptible to these disorders include individuals over 50 years old,9 postmenopausal women,6 overweight individuals,10 sedentary people,11 and athletes.12
Nutritional interventions, including dietary supplements use, are commonly employed in the management of musculoskeletal disorders.9 Collagen supplementation may support the treatment as the protein is ubiquitous in connective tissues, consisting of around 30% of human proteins. While there are many types of collagen proteins, type I collagen is the most abundant among them. Type I is primarily synthesized by fibroblasts and osteoblasts and found predominantly in tendons, ligaments, and bones.7,8 The benefits of collagen intake for conditions that affect the musculoskeletal system have been suggested by previous works.13–15
The collagen bioavailability is influenced by the supplement form. Type I collagen hydrolysate is a dietary supplement composed of low molecular weight peptides (<6 kDa) derived from native collagen through heat denaturation and enzymatic hydrolysis.16,17 This complex multi-step hydrolysis results in biologically active collagen peptides that stimulate the metabolism of collagen-producing cells. Collagen peptides or hydrolyzed collagen (HC) offer significant advantages over integral protein forms, such as undenatured collagen, including higher digestibility and absorption rates.18–20
Recent collagen reviews have explored the specific effects of collagen supplementation in osteoarthritis, joint injury, and muscle recovery.13–15 However, no recent reviews have focused on type I collagen hydrolysate. Hence, the study objective is to summarize the literature on the effects of type I collagen hydrolysate supplementation on the musculoskeletal system, specifically regarding bones, muscles, and joints.
Methods
The searches were conducted in August 2024. The following databases were used: PubMed (MEDLINE), Scopus, EMBASE, and CINAHL. Search strategies were partially constructed using the PICO process (population, intervention, comparison, outcome) and employed terms related to the study’s objective, combined with Boolean operators “OR” and “AND”. The following terms were used: supplement, oral, nutraceutical, chondroprotec, complement, administration, gelatine, procollagen peptide, collagen, procollagen, ‘hydrolyzed collagen’, ‘collagen hydrolysate’, musculoskeletal, muscle, joint, cartilage, ligament, fascia, skeletal, bone. The operator “*” was added to capture variations of terms. In PubMed, search terms were applied in the fields text word, MESH terms, and title. In EMBASE, search terms were applied in the field TITLE-ABS-KEY. Filters for systematic review and randomized controlled trials (RCT) were applied in the respective databases. The search period was from 2000 to August 2024.
Inclusion criteria were RCTs or systematic reviews that investigated oral supplementation of type I collagen hydrolysate, which collagen was administered in isolated form. Exclusion criteria were pre-clinical studies, experimental studies, different types of collagens or non-hydrolyzed collagen, studies combining collagen with other ingredients, studies with beauty-related endpoints, studies not-focused on the musculoskeletal system, and unblinded, nonrandomized, and uncontrolled clinical trials. The search results were added to the Rayyan, a reviewer tool. Duplicate papers and articles without access to full text were excluded. Reviews were included to identify additional studies that might not have been captured by the primary search strategy.
After assessing the eligibility, the studies were grouped according to the main component of the musculoskeletal system the study focused on (bones, joints, and muscles) and the population studied (healthy individuals, patients with osteoarthritis, or individuals with muscle/joint pain).
To obtain an objective metric for the results of the included studies, the Positive Outcome Rate (POR) was applied. The metric was calculated by dividing the number of outcomes with significant improvement (p<0,05) in the intervention group by the total number of outcome measures investigated. A POR value of 1,0 represents the highest possible score, representing an improvement in all studied outcomes.
Results
The search strategy identified 6042 studies of which 35 RCTs were included in the review. The search workflow is depicted in Figure 1. Additionally, four systematic reviews were found and assessed for eligibility. Of these, two studies did not meet the inclusion criteria, one could not be assessed, and one report met the criteria. In total, 36 RCTs were included in the review. Study characteristics are detailed in Table 1.
All studies were double-blind, except in two studies: one was only investigator-blinded and the other was participant-blinded. The majority of studies were placebo-controlled, except four that employed parallel groups to evaluate different supplementation doses or protein sources. Four studies utilized crossover designs.
Regarding the sample composition, 16 studies included only male individuals, 7 included only female participants, and 13 studies had mixed-sex samples. In terms of age, 18 studies had participants 18-40 years, nine included participants aged ≥50 years, and the remaining nine were mixed aged groups. Regarding health conditions, 20 reports included healthy samples while 16 focused on various pathologies.
Collagen supplementation doses ranged from less than 5g/day to over 20g/day: 13 studies used 15g/day, eight studies used 10g/day, seven studies used above 20g/day, five studies used 5g/day, and two studies used less than 5g/day. One study did not report the dose. The majority of studies used maltodextrin or silica complex as a placebo, with three reports using whey protein as a comparator.
The average duration of the studies was 15±11.4 weeks, with a maximum duration of 52 weeks (equivalent to one year) and a minimum duration of 0.15 weeks (equivalent to one day). The mean POR of the included studies was 0.4, indicating fewer than half achieved a significant difference in the evaluated outcomes. The average dropout rate was 17.4±10.9 % with an average of 76±57.7 participants per study.
Studies Focused on Bones
Three reports investigated bone-related endpoints. Two studies were on postmenopausal women with osteopenia at a mean age of 60,8 years. One study investigated a healthy male sample at a mean age of 24 years. The supplementation dose varied from 5g/day-20g/day of collagen. The study period ranged from three days to one year. The mean drop-out was 19,9%. The studies used bone formation (osteocalcin (OSCAL), bone-specific alkaline phosphatase (BAP), amino-terminal propeptide of type I collagen (P1NP)) and bone reabsorption (carboxyl-terminal collagen cross-links (CTX), Type I collagen C-telopeptide (CTX 1)) biomarkers to evaluate the endpoints. One study also evaluated bone mineral densitometry (BMD) of the femoral neck and spine between L1 and L4. The endpoints and main results of the studies are summarized in Table 2.
Cúneo, F. et al.6 sought to analyze the performance of bone biomarkers in post-menopausal women with collagen supplementation for 24 weeks. Although BAP and CTX demonstrated differences in their levels during the study period, the difference between groups was not significant. König, et al.26 designed the study with 5g collagen in a period of one year with a similar sample, also evaluating BMD. P1NP significantly increased in the collagen peptides (CP) group, whereas CTX 1 increased in the placebo group during the period, but the authors did not compare the activity between groups. BMD in the spine and femoral neck had differences between study groups indicating an increase in bone density in the collagen group. However, the groups demonstrated baseline differences for body mass index and BMD spine. Hilkens L, et al.43 associated collagen supplementation with high-impact exercise in healthy young males. However, both groups demonstrated a difference in bone biomarkers and the study period was three days.
Studies Focused on Joints
Fourteen studies assessed the effect of collagen on joints. Of these, two included healthy volunteers, while the remainder focused on individuals with joint pathologies. The pathologies were primarily joint pain, covered in five articles, followed by OA, addressed in five articles, and finally, one study examined participants with chronic knee instability, and one with volunteers suffering from calcaneal tendinitis.
The collagen doses varied between 1,2-20 g/day. Five studies used 5g/day, five used 10g/day, one used 1,2g/day, one used 2,5g/day, one used 8g/day, and one used 20g/day. The trials were conducted between 2011 and 2024, with an average duration of 19,5±10,8 weeks. The total number of participants was 1.652, resulting in an average of 110±71 participants per study with an average dropout rate of 11,3±10,3%.
Outcomes were the most frequently accessed by the Visual Analogue Scale (VAS), cited in 6 articles. Followed by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the Knee Injury and Osteoarthritis Outcome Score (KOOS), both of which were used in 3 of the 14 analyzed articles. The rate of positive outcomes in studies examining the effects of collagen on joints was 0.49±0,3. The endpoints and main results of the studies are summarized in Table 3.
Zdzieblik et al.25 observed a POR of 0.6 in the assessment of pain reduction in athletes with functional knee problems during sports activities associated with specific collagen peptides (SCP) use. Pain intensity was assessed using VAS and additional therapies demonstrated a statistically significant improvement in activity-related pain. Bongers et al.36 reported no superior effect of collagen compared to placebo. Supplementation with both treatments resulted in similar reductions in knee pain and improvements in knee function after 12 weeks.
Studies focused on Muscles
Nineteen articles addressing the collagen effects on muscle strength and body composition. Of these, only one study focused on pathological volunteers. The mean dosage of collagen peptides used was 20g/day. The study durations ranged from less than a day to 15 weeks. The endpoints and main results of the studies are summarized in Table 4.
Zdzieblik D et al.39 investigated the effects of daily supplementation with SCP compared to whey protein and placebo, within the context of a 12-week resistance training program. The study sought to determine whether CP could induce a significant increase in lean mass and a greater reduction in body fat compared to the other groups, especially whey protein. The findings revealed that SCP supplementation, in combination with resistance training, resulted in a significant increase in lean mass and a substantial reduction in body fat compared to the placebo. Whey protein supplementation positively impacted lean mass but did not differ from the placebo. There was no significant difference between the effects of CP and whey protein concerning lean mass gains.
Previous research by the same author24 demonstrated that a 12-week exercise protocol combined with collagen supplementation further improved body composition in elderly, sarcopenic men by increasing lean body mass, muscle strength, and reducing fat mass. Additionally, another study29 aimed to explore the effects of resistance exercise combined with SCP supplementation on body composition and muscle strength in premenopausal women. The treatment group (TG) exhibited a statistically significant increase in the percentage of lean mass compared to the control group (CG). The women in the study also had a significant decline in overall body fat percentage, with greater body fat loss in the TG.
At the molecular level, Oertzen-Hagemann31 evaluated the outcomes of a 12-week hypertrophy protocol in men aged from 21-27 years, combined with a daily 15g of SCP supplementation. The objective was to assess the impact of this protocol on muscle proteomics. Although results indicated that the collagen-supplemented group exhibited a more pronounced effect on muscle-related proteins and pathways associated with resistance training adaptation, the findings were not entirely clear. Furthermore, the author found that participants in the intervention group experienced an increase in body mass and lean body mass, with no significant difference in fat mass between groups.
Jerger and colleagues44 investigated whether collagen supplementation combined with high-load resistance exercise could upregulate gene expression in pathways involved in skeletal muscle transduction. The study assessed gene expression in the PI3K-Akt, MAPK, and mTOR pathways through mRNA analyses at various time points following exercise. Results demonstrated a significant upregulation of gene expression in the PI3K-Akt and MAPK pathways 4 hours post-exercise in the collagen group. However, there were no significant differences in mTOR pathway expression between the groups. Despite 100% adherence from participants in the study, the evaluation period and sample characteristics limit broader generalization.
Discussion
The purpose of this systematic review was to evaluate the effects of type I HC supplementation on bones, joints, and muscles. Our findings indicate that HC intake might enhance different outcomes in the musculoskeletal components. However, the heterogeneity in study designs, methodological limitations and lack of reporting items, result in an inconsistency of results between studies.
Studies that evaluate bone outcomes were the fewest compared to joints and muscles. All studies presented methodological issues, such as outcomes evaluation, or limitations, such as study period and population sample, that prevent generalizations about the effect of collagen supplementation on bone health. The evaluation of these outcomes in healthy populations is challenging, especially in younger individuals, as the modification of serum biomarkers or BMD is more likely in the occurrence of pathologies. Cúneo, F. et al.6 and König, D. et al.26 demonstrated changes in bone biomarker levels in similar populations, postmenopausal women, over different study periods. However, the study groups did not have clear differences, and the modifications mostly occurred despite the collagen intake. The results presented by König, D. et al.26 indicate that collagen supplementation might assist in the increase of BMD of the femoral neck and lumbar spine in postmenopausal women with osteopenia.
The majority of articles evaluating the effects of collagen supplementation on joint health reported beneficial outcomes, including reductions in joint pain, improvements in clinical parameters,9 increased physical mobility,23 and enhanced ankle function12 (Table 3). Jiang et al,23 in their study involving women with moderate knee OA, as well as Zdzieblik et al25,38 in two studies examining the relationship between collagen supplementation and physical exercise, observed significant improvements in joint pain. A hypothesis for the improvement in pain could be the reduction of inflammation. It is suggested that collagen may have anti-inflammatory properties, and supplementation could thus reduce the production of inflammatory markers, thereby improving joint function.25 The highest number of significant results for HC supplementation in joint outcomes was observed in subjectively reported outcomes22,23,49 (Table 3). However, the lowest rates of positive outcomes (POR = 0.25) were found in studies that used more objective and precise outcome measures, such as Cartilage Magnetic Resonance Imaging (dGEMRIC), B-mode ultrasound, and specific tests like the McMurray test, Steinmann test, and Drawer test.21,38,41
Collagen supplementation is suggested to improve the functional, structural, and contractile aspects of skeletal muscle. Even though our search found studies demonstrating positive effects of collagen supplementation in body composition, the effectiveness of the supplement in increasing muscle mass varies, with some studies demonstrating positive results and others not confirming this effect. Using proteomics, Oikawa et. al.34,35 showed in two studies that α-lactalbumin and whey were superior to collagen in increasing muscle protein synthesis. In one study, the author showed that myofibrillar and sarcoplasmic proteins, in trained individuals who consumed α-lactalbumin, had superior rates when compared to individuals who consumed collagen peptides, suggesting that a higher protein quality plays a key role in muscle remodeling. Similarly, the same author presented that whey protein had higher protein synthesis rate, corroborating with the anterior result. In addition, Balshaw et al42 did not find strength gains in subjects who took collagen peptides after a 15-week resistance training protocol. These results reinforce the need for further studies using collagen peptides and their effects on muscle gains.
Our findings are in line with the results found by Khatri et al, 2021.14 The authors conducted a systematic review about collagen supplementation on body composition, collagen synthesis, and recovery from joint injury and exercise. Improving joint functionality and reducing joint pain were demonstrated as the most beneficial. Changes in body composition, especially in FFM, had improvements when combined with resistance training. Collagen supplementation indicated an increase in collagen synthesis which coupled with an intermittent exercise protocol may improve tissue repair and prevent injuries. Regarding muscle protein synthesis, other high-quality protein sources, such as whey protein, may be more indicated.14 These findings reinforce the potential of collagen supplementation while acknowledging its limitations compared to other protein sources.
In general, studies presented limitations regarding the duration of the intervention. The mean study period was 15 weeks ranging from 1 day to 1 year (Table 1). Most studies reported significant positive differences after 12 weeks (3 months) or more. Shorter periods of evaluation hardly demonstrated significant differences with only the supplementation use. Certain outcomes due to the physiology of the musculoskeletal system components demand long periods and consistency of intervention to demonstrate substantial differences, such as bone activity.
The studies do not present standardization in dose administration of collagen supplement. The high degree of variability in dosage use impacts the generalization of findings. Additionally, the majority of studies used commercially available collagen supplements without specifying the collagen type. Most reports cited the brand composition of the collagen, which presents another limitation for generalization as different brands do not have the same amino acid composition even if it is the same type of collagen. The lack of trial design and reporting standardization emerges as obstacles for definitive conclusions regarding the optimal dosage for specific musculoskeletal outcomes.
Most studies had small sample sizes, having 15 studies with a sample size above 50 participants. This feature might generate high variation between studies enabling the occurrence of conflicting findings. The rate of dropouts in the studies cannot be ignored. While a drop-out rate <20% is acceptable, 14 studies reported a rate above the acceptable. Studies with long periods of intervention are more likely to lose participants’ adhesion, especially when participants cannot perceive substantial benefits from the intervention.
Conclusion
Achieving clinically and statistically significant results is particularly challenging in areas with inconsistent prior research and lack of standardized testing methods, such as evaluating the benefits of collagen as a dietary supplement for joint, muscle, and bone health. Additionally, the multifactorial complexity of these studies and conditions evaluated present another obstacle to drawing definite conclusions regarding the effects of collagen supplementation. Even so collagen supplementation demonstrated positive effects compared to placebo or alternative interventions particularly in high-risk groups, such as elderly adults and individuals with pre-existing joint-disorders, while having a favorable safety profile.
Our review revealed dramatic heterogeneity in study protocols, including differences in sample size, study population, duration, and outcome measurements. The diversity of concentrations and dosages between studies, added to the lack of adverse events (AE) indicate a probable security of collagen supplement. However, it is not clear if patients did not present AEs, or the authors did not report or evaluate.
The dropout rate in several studies exceeded 20%, suggesting that study design or participant adherence may need to be reevaluated. This high rate may have affected sample sizes and compromised the validity of the findings. Additionally, while validated, self-reported outcome measures like VAS may introduce subjectivity. Future research could prioritize more objective assessments, such as MRI, BMD, and ultrasound to enhance the reliability of results.
The relatively short duration of many studies might have contributed to the lower POR, as insufficient time may have prevented substantial physiological changes from being induced. Future research should design study protocols with longer periods of intervention. Furthermore, the commercial funding of most studies raises concerns about potential conflicts of interest. Authors need to clearly state their affiliations, funding and potential conflicts of interest.
Despite these limitations, some studies reported promising results, such as increased BMD, which could have significant implications for clinical practice. Notably, changes in body composition were prominent in studies involving physical exercise and healthy volunteers. Future research should explore whether similar outcomes can be observed in pathological populations, with or without accompanying training protocols. Finally, patients suffering from joint pain could potentially benefit from collagen supplementation if future research provides robust evidence demonstrating its effectiveness in alleviating symptoms.
Contributions of each author
Paula J Brueckheimer, Tales C Silva and Leonardo B Rodrigues: planning, data analysis or interpretation and writing of the article.
Carlos Isaia Filho: conception, planning, critical intellectual review, and responsibility for final approval for publication.
Vivian Zague: critical intellectual review, and responsibility for final approval for publication.
Conflicts of Interest
The authors declare that they have no conflicts of interest related to this systematic review. However, it is important to note that one of the authors (VZ) is employed by Genu-in, a company that produces hydrolyzed collagen. This affiliation does not influence the findings or conclusions of this review, which are based solely on the analysis of the available literature.
Funding
Genu-in covered the article submission fees