Introduction
Anterior cruciate ligament (ACL) injuries are among the most common and debilitating musculoskeletal injuries in athletes, leading to prolonged rehabilitation and often incomplete recovery of dynamic knee stability. While the structural damage to the ligament is a primary concern, ACL injuries also result in profound neurophysiological changes that disrupt the sensorimotor control of the knee.1 The ACL is not merely a passive stabilizer; it is an active sensory organ integrated with the central nervous system (CNS), contributing to the dynamic regulation of knee movements through its dense network of mechanoreceptors. These sensory receptors provide critical proprioceptive feedback that informs the CNS of joint position, tension, and motion, coordinating neuromuscular responses to protect the knee from excessive loading.2 When the ACL is injured, this complex sensorimotor system is disrupted, leading to deficits in proprioceptive accuracy, delayed muscular reflexes, and altered motor control strategies.3
These disruptions extend beyond the knee joint, affecting the entire neuromuscular system, and are compounded by neuroplastic changes in the CNS, including altered cortical activation patterns and impaired sensory integration.4 Rehabilitation strategies that focus solely on restoring strength and stability at the knee often fail to address these complex neurophysiological deficits, resulting in incomplete recovery and a high risk of re-injury.5 Traditional rehabilitation approaches may be insufficient to rewire the altered neural circuits, necessitating a more comprehensive strategy that leverages the principles of neuroplasticity to restore optimal sensorimotor function.6
Neuroplastic therapy, a cutting-edge approach in ACL rehabilitation, capitalizes on the brain’s ability to reorganize itself through targeted exercises and advanced tools such as external focus techniques, stroboscopic glasses, smartboards, and virtual reality (VR).7 These tools challenge the CNS to adapt to varying levels of sensory input and motor complexity, promoting the re-establishment of efficient, automatic motor patterns and enhancing neuromuscular resilience. This integrative approach aims to restore not only joint stability but also the underlying neural networks that support dynamic movement control.8 By addressing both the peripheral and central components of ACL injury, neuroplastic therapy represents a paradigm shift in rehabilitation, offering a more holistic solution to the complex challenge of restoring full functional performance and reducing re-injury risk.
1. Neurophysiology of ACL injuries
The anterior cruciate ligament (ACL) is not merely a passive stabilizer of the knee but rather a complex structure that integrates deeply with the central and peripheral nervous systems, making it a key player in the body’s sensorimotor framework.9 This integration is crucial for maintaining dynamic knee stability and coordinated movement. Early observations by Payr revealed that the ACL houses a sophisticated network of sensory receptors, including Ruffini endings, Pacinian corpuscles, and Golgi tendon organs. These specialized mechanoreceptors detect mechanical deformation such as stretch, tension, and compression within the ligament.10 Once activated, these receptors send detailed proprioceptive information to higher centers in the spinal cord and brainstem, where it is processed and translated into motor commands that regulate joint stability.11
This sensory input is not only vital for immediate reflexive responses but also for shaping the body’s long-term motor strategies.12 For example, when a sudden perturbation occurs, such as a rapid forward shift of the tibia relative to the femur, the afferent signals from the mechanoreceptors rapidly ascend to the CNS and initiate a protective reflex arc. This neural pathway, known as the “ligamento-muscular protective reflex,” triggers a rapid contraction of the hamstring muscles, countering the anterior tibial translation and reducing the load on the ACL.13 This monosynaptic reflex pathway, which involves direct communication between sensory neurons in the ligament and alpha motor neurons controlling the hamstrings, allows for an extremely quick response time, which is essential for joint protection under sudden, high-force conditions (Figure 1).14
The involvement of the MCL and other knee ligaments in similar reflex arcs suggests that the ligamento-muscular system is a comprehensive, multi-ligament network designed to provide synergistic stabilization across multiple planes of motion.15 This system ensures that not only the primary stabilizers such as the hamstrings are activated but also the secondary muscle groups, such as the sartorius and the vastus medialis, providing a coordinated defense against potentially harmful forces.16 This multi-muscle activation pattern is necessary for effective dynamic knee stability, as it helps distribute the load across several muscle groups, reducing the stress on any single structure.17
The nature of the neuromuscular connections between the ACL and the surrounding musculature can be further understood by examining animal models.18 Studies in felines have demonstrated that when the ACL is artificially loaded, the resulting mechanoreceptor activation leads to a rapid and robust contraction of the surrounding muscles, effectively preventing ligamentous failure. This reflex arc can be mimicked in humans through the direct electrical stimulation of the ACL, highlighting a well-defined neuromuscular circuitry.19 This circuitry suggests that the ACL, far from being an isolated stabilizing structure, plays a central role in the real-time regulation of knee joint movement through its intimate connections with the sensorimotor system.20
Beyond the protective monosynaptic reflex, the interplay between ligamentous and muscular mechanoreceptors reveals a sophisticated network of feedback and feedforward mechanisms that regulate muscle stiffness and contraction strength.21 The ligament-spindle reflex, for example, involves the muscle spindles located within the muscles adjacent to the knee. These spindles are sensitive to changes in muscle length and the speed of stretch, providing an additional layer of feedback control. When a muscle spindle is rapidly stretched, it generates a high-frequency burst of action potentials that are transmitted via sensory neurons to the spinal cord.22 Here, these signals synapse directly onto motor neurons that innervate the same muscle, causing an immediate contraction to resist further elongation. This protective response helps maintain muscle length-tension relationships and prevents the joint from exceeding its safe range of motion.23
In the context of ACL strain, both the ligamento-muscular reflex and the muscle-spindle reflex work together to stabilize the knee.24 When the ACL experiences anterior tibial translation, the muscle spindles within the hamstring muscles are activated almost simultaneously, creating a dual mechanism of protection.25 This ensures that not only is the tibial motion resisted, but the entire kinetic chain is adjusted to prevent excessive anterior translation and potential ligament rupture.26 This duality is especially critical during rapid, high-energy movements such as cutting or jumping, where the forces acting on the knee can reach levels that are difficult to control through voluntary muscular action alone.27
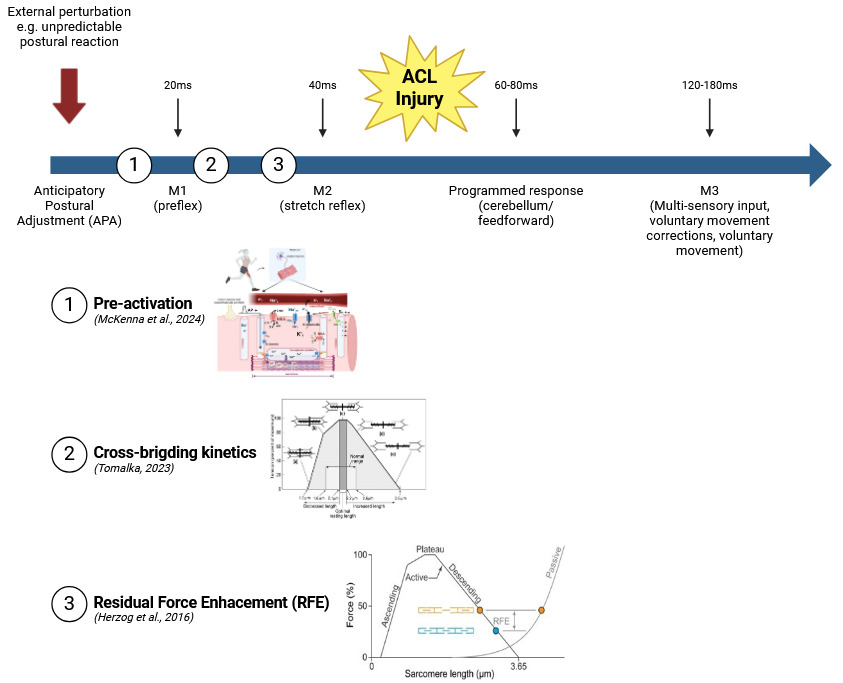
However, despite these advanced protective mechanisms, there are limitations to the speed and efficacy of reflex responses.28 Non-contact ACL injuries, which typically occur within 17 to 60 milliseconds after initial foot-ground contact, present a challenge for reflex-based protection.29 With a typical reflex latency of approximately 70 milliseconds, there is simply not enough time for the ligamento-muscular reflex to engage fully before the ACL is subjected to damaging forces.30 This timing discrepancy highlights a critical gap where reflexive responses alone are insufficient to prevent injury. Instead, pre-activation of the surrounding musculature becomes a crucial factor. Pre-activation involves the anticipatory contraction of muscles before foot contact, enhancing joint stiffness and preparing the knee for incoming forces.31 This proactive neuromuscular strategy is crucial for athletes, as it allows for greater control over knee stability even before the mechanical load is applied.32
In individuals with ACL deficiency or rupture, the absence of these protective reflex pathways leads to significant neuromuscular deficits.33 Without the proprioceptive feedback from the ACL, the CNS cannot accurately detect changes in joint position and loading, resulting in delayed and uncoordinated muscular responses.34 Studies have shown that ACL-deficient patients exhibit a markedly slower hamstring activation time compared to their intact counterparts, leading to compromised dynamic stability and an increased risk of secondary injuries.35 This altered neuromuscular response is indicative of a reorganization within the CNS, where other sensory inputs, such as visual or cutaneous cues, are recruited to compensate for the lost ligamentous feedback.36
Yet, these compensatory mechanisms are often inadequate during high-speed or unpredictable movements, leading to a heightened risk of instability.37 This suggests that rehabilitation in ACL-deficient individuals must prioritize not just strength training, but also the restoration of neuromuscular control through targeted proprioceptive exercises and motor learning interventions. The goal is to reestablish efficient sensorimotor pathways that can provide real-time joint stability even in the absence of direct ligamentous input.38 Techniques such as perturbation training, where unexpected shifts in weight or balance are introduced during exercises, can help retrain the CNS to respond more effectively to sudden changes in joint position.39
Furthermore, neuroplastic changes in the CNS following ACL injury can contribute to altered motor control strategies.40 Studies using functional MRI have shown that ACL-deficient patients demonstrate increased activation in the motor cortex and supplementary motor areas during knee movements, suggesting a shift toward more conscious motor control strategies.41 This increased cortical involvement likely represents a compensatory adaptation to the loss of automatic, reflexive control, but it also places additional cognitive demands on the patient during movement, increasing the risk of fatigue and errors during complex motor tasks.42 Rehabilitation should therefore focus not only on restoring peripheral proprioceptive function but also on promoting adaptive neuroplasticity within the CNS to optimize motor learning and performance.43
Understanding these complex neurophysiological mechanisms is essential for developing effective prevention and rehabilitation strategies.44 It emphasizes the need to view the ACL as an active participant in joint stability, deeply embedded in a larger sensorimotor network that integrates sensory feedback, motor control, and neuromuscular coordination.45 Such an integrated approach can lead to more comprehensive treatment plans that not only restore physical function but also rewire the underlying neural networks to prevent future injuries.
2. Motor Control of Knee Joint: ACL Perspective
The brain regions involved in motor control undergo significant neurophysiological changes following an anterior cruciate ligament (ACL) injury, affecting not only sensory and motor processing but also the overall neuromuscular coordination of movement (Figure 2).46 Each labeled structure in the brain corresponds to alterations in motor control strategies that emerge as the central nervous system (CNS) adapts to the disrupted proprioceptive inputs and sensorimotor deficits caused by the injury.47
The frontal gyri, located in the frontal lobe, are integral to higher-order cognitive processes such as motor planning, decision-making, and voluntary movement control.48 In normal conditions, this region facilitates the transition from deliberate movements to more automatic, reflexive actions through motor learning and practice. Post-ACL injury, there is an observable increase in activation within the frontal gyri, indicating a shift from subconscious motor execution to a more conscious and effortful control of movement.49 This heightened activation reflects the brain’s compensatory strategy to account for impaired proprioceptive feedback and altered knee joint stability. Individuals rely more on cognitive resources to monitor and control knee movements consciously, leading to increased mental fatigue and slower motor responses during complex tasks.50 Movements that were once automatic, such as cutting or changing direction, now require active planning and attention, which can impair performance and increase the risk of errors.
The contralateral motor cortex, responsible for executing voluntary movements on the opposite side of the body, shows altered activation patterns after an ACL injury.51 Typically, efficient motor control involves streamlined processing through established neural circuits with minimal cortical input for well-practiced movements. Following injury, the contralateral motor cortex exhibits increased activation during both simple and complex motor tasks.52 This change reflects the brain’s effort to restore precise motor control in the affected limb by increasing cortical involvement. The heightened activation suggests that motor commands are more reliant on direct cortical input rather than subcortical or spinal pathways.53 This reliance can lead to increased muscle co-contraction around the knee as the CNS attempts to stabilize the joint through simultaneous activation of agonist and antagonist muscles.54 While co-contraction can enhance joint stiffness and stability, it may also result in reduced movement efficiency and flexibility, making dynamic activities more challenging and potentially increasing the risk of compensatory movement patterns that could lead to further injury.
In typical movement control, the ipsilateral motor cortex (on the same side as the moving limb) plays a secondary role, primarily involved in bilateral coordination and complex bimanual tasks.55 However, after an ACL injury, the ipsilateral motor cortex becomes more active, indicating bilateral cortical recruitment to enhance motor control of the injured limb.56 This increased activation suggests that the brain is utilizing both hemispheres to send redundant signals to the affected muscles, potentially as a means to compensate for weakened or unreliable neural pathways.57 While this bilateral activation may temporarily enhance joint stability, it can also signify an over-reliance on conscious control mechanisms, reducing the CNS’s ability to respond rapidly and efficiently to unexpected perturbations or high-speed movements.58 The increased cognitive load associated with bilateral cortical activation can lead to mental fatigue and decreased motor performance over time.
The intraparietal sulcus, part of the parietal lobe, is heavily involved in integrating sensory information and coordinating motor actions, especially for visually guided tasks.59 Post-ACL injury, increased activation in this region indicates a greater reliance on visual inputs to compensate for diminished proprioceptive feedback from the knee joint.60 Individuals may adopt a visually dependent strategy to guide movements, using visual cues to estimate limb position and joint angles. This shift can slow down reaction times during dynamic activities, as visual processing is slower than proprioceptive feedback.61 Moreover, over-reliance on visual guidance can impair the automaticity and fluidity of movement patterns essential for high-level athletic performance.62 The increased cognitive demand to process visual information can also divert attention from other critical aspects of movement, such as spatial awareness and anticipatory adjustments.
The lingual gyrus, located in the occipital lobe, is primarily responsible for processing visual information related to complex patterns and spatial orientation.63 Following an ACL injury, heightened activation in the lingual gyrus reflects the brain’s attempt to use visual feedback as a substitute for lost proprioceptive input.64 While this compensation strategy demonstrates the brain’s adaptability, it is less effective during high-speed or unpredictable movements where rapid adjustments are necessary. Over-reliance on the lingual gyrus can hinder dynamic motor control, making it difficult for individuals to adjust movements quickly in response to unexpected changes in the environment.65 This can increase the risk of re-injury or impair performance in activities requiring quick reflexes and precise coordination.
The secondary somatosensory cortex (SII) processes complex sensory inputs related to touch and proprioception, integrating information from both sides of the body.66 After an ACL injury, increased activity in the SII suggests that the brain is working harder to integrate altered sensory inputs from the injured knee.67 This increased demand reflects the need to reprocess and reinterpret sensory information, often relying on other sensory modalities such as cutaneous (skin) or visual inputs to compensate for disrupted proprioceptive feedback.68 This reorganization may lead to sensory misinterpretation, where the brain’s perception of joint position and movement does not align with the actual mechanical state of the knee.69 Such a sensory mismatch can contribute to altered motor control strategies, resulting in slower or inaccurate responses to perturbations and an increased risk of joint instability.70 The brain’s effort to reconcile conflicting sensory information can also increase cognitive load, further complicating motor control.
The pre-supplementary motor area (pre-SMA) is critical for initiating complex, multi-step movements and adapting motor plans in response to changing conditions.71 Increased activation in the pre-SMA post-ACL injury indicates a shift toward more deliberate and effortful movement planning.72 Movements that were previously executed with minimal conscious effort now require active cognitive oversight, reflecting a loss of automaticity in motor control.73 This shift can impair the fluidity of movement transitions and increase the risk of movement hesitations or errors, particularly during tasks that demand rapid changes in direction or speed.74 The increased cognitive load associated with pre-SMA activation can lead to greater mental fatigue and reduced endurance during prolonged or high-intensity activities.
The posterior inferior temporal gyrus (pITG) is traditionally associated with visual object recognition and complex visual processing.75 Following an ACL injury, increased activation in the pITG suggests a compensatory reliance on visual-spatial processing to guide knee movements.76 This adaptation can result in an overemphasis on limb positioning and visual monitoring, disrupting the balance between visual and proprioceptive inputs. While this strategy may help maintain some degree of movement control, it can lead to stiffness and overly cautious movements.77 The visual system is less capable of providing the rapid, real-time feedback necessary for dynamic and reflexive motor adjustments compared to proprioceptive pathways.78 Consequently, reliance on the pITG may impair the ability to perform quick, agile movements and respond effectively to sudden changes in the environment.
The cerebellum plays an essential role in motor coordination, balance, and timing of movements by adjusting and refining motor actions based on sensory feedback.79 After an ACL injury, the cerebellum shows altered activation patterns, indicating increased effort to adapt to disrupted sensory signals from the knee joint.80 This heightened cerebellar activity suggests that the brain is attempting to recalibrate the internal models that guide knee movements, which rely on accurate integration of sensory and motor information.81 Without reliable proprioceptive input, the cerebellum may struggle to make precise adjustments, leading to increased variability in joint control and a greater risk of instability during dynamic activities.82 Enhanced cerebellar activation reflects the brain’s effort to re-learn stable movement patterns, emphasizing the importance of incorporating cerebellar-based training in rehabilitation programs to restore efficient motor control.83
Overall, the neurophysiological changes observed in these brain regions reflect a fundamental shift from efficient, automatic motor control to a more effortful and cognitively demanding strategy.84 This altered motor control pattern is a protective response to the disrupted sensorimotor feedback following an ACL injury. However, it can impair performance and increase injury risk if not addressed through targeted rehabilitation strategies.85 The increased cognitive load and reliance on conscious control mechanisms can lead to mental fatigue, slower reaction times, and decreased movement efficiency. To counteract these changes, rehabilitation should focus on restoring proprioceptive function, enhancing automaticity in motor control, and reintegrating efficient neural pathways to optimize movement patterns and reduce the risk of re-injury.
3. Acute Molecular Changes Immediately After ACL Injury.
During the acute phase of an ACL injury, the rapid influx of blood components and inflammatory mediators into the joint space profoundly impacts local cellular behavior and shapes subsequent neurophysiological responses that drive arthrogenic muscle inhibition (AMI) (Figure 3).86 Vasodilation and increased vascular permeability, triggered by bradykinin, histamine, and prostaglandins, allow immune cells such as neutrophils, monocytes, and macrophages to enter the synovial compartment.87 These cells, activated by cytokines like interleukin-1 beta (IL-1β) and interleukin-6 (IL-6), release additional inflammatory signals that act in a feedforward manner to exacerbate joint swelling and pain perception.88 At a molecular level, pro-inflammatory cytokines interact with toll-like receptors (TLRs) and their downstream adaptor proteins (e.g., MyD88, IRAKs, TRAF6) to activate nuclear factor-kappa B (NF-κB).89 Once translocated to the nucleus, NF-κB drives the expression of more cytokines, chemokines, and matrix metalloproteinases (MMPs), perpetuating a catabolic environment that undermines the structural integrity of the extracellular matrix (ECM).90
As MMP-3 (stromelysin-1) and MMP-13 (collagenase-3) become overexpressed, collagenous and proteoglycan components of the ECM are progressively degraded.91 This process, if unchecked, can compromise not just the injured ACL but also articular cartilage and supporting tissues, intensifying instability in the joint.92 From the standpoint of AMI, ongoing ECM breakdown and inflammation continuously stimulate group III (Aδ) and group IV (C) afferent fibers.93 These small-diameter nociceptors, already sensitized by prostaglandin E2 (PGE2) and bradykinin, relay amplified signals to the dorsal horn of the spinal cord, where they release glutamate and neuropeptides like substance P.94 The heightened activation of N-methyl-D-aspartate (NMDA) and AMPA receptors in dorsal horn neurons further potentiates nociceptive pathways, inducing central sensitization and reshaping local interneuron networks.95
Cytokines such as IL-1β and IL-6, once diffused into the spinal cord or transported across a more permeable blood-brain barrier, modulate synaptic transmission by altering neurotransmitter release and receptor expression.96 IL-1β can upregulate excitatory glutamatergic signaling while concomitantly reducing inhibitory gamma-aminobutyric acid (GABA)ergic tone.97 This imbalance translates into a net excitatory shift in pain circuits, enhancing pain perception but paradoxically promoting stronger inhibitory reflex arcs (via spinal interneurons releasing GABA and glycine) onto alpha motor neurons that innervate the muscles around the injured knee.98 In the context of AMI, such modulation is protective in the short term—reducing force output and protecting the ACL from further stress—but it risks persistent muscle inhibition, atrophy, and weakness over time.99
Microglia and astrocytes in the spinal cord further exacerbate this shift when exposed to pro-inflammatory signals.100 Activated microglia produce cytokines like IL-1β and tumor necrosis factor-alpha (TNF-α), intensifying dorsal horn neuron excitability.101 Astrocytes release gliotransmitters, including glutamate and ATP, that reinforce hyperexcitable states in nociceptive pathways and support inhibitory synapse plasticity targeting alpha motor neurons.102 This interplay between neuronal and glial cells locks the spinal cord into a pattern of amplified pain processing coupled with heightened inhibitory drive to the quadriceps, particularly the vastus medialis oblique (VMO).103 Over time, repeated activation of NF-κB or signal transducer and activator of transcription (STAT) proteins in these glial populations can alter the expression of ion channels, receptor subunits, and growth factors, perpetuating a cycle of abnormal synaptic remodeling.104
Myostatin, a key negative regulator of muscle growth belonging to the transforming growth factor-beta (TGF-β) superfamily, may become upregulated in the catabolic and disuse conditions that frequently follow ACL injury.105 Myostatin binds to activin type IIB receptors (ActRIIB) on muscle cells, triggering SMAD2/3 phosphorylation and suppressing myogenic regulatory factors such as MyoD and myogenin.106 In the inflamed joint environment, heightened pro-inflammatory cytokines can further potentiate myostatin’s downstream effects, reducing muscle protein synthesis and exacerbating quadriceps atrophy.107 This atrophic process aligns with the reflexive inhibition of motor neurons, leading to pronounced AMI and complicating rehabilitative efforts.108
In parallel, reactive oxygen species (ROS) produced by activated immune cells can overwhelm local antioxidant defenses.109 Manganese superoxide dismutase (MnSOD), an enzyme localized mainly in the mitochondria, catalyzes the dismutation of superoxide radicals into hydrogen peroxide and oxygen.110 Although MnSOD expression often increases in response to oxidative stress, sustained inflammation and repeated oxidative bursts may exceed the enzyme’s protective capacity.111 This excess oxidative stress can further damage synovial cells, chondrocytes, and myocytes, disrupting energy metabolism and amplifying inflammatory signaling pathways (e.g., via NF-κB and MAPK).112 Under such conditions, additional upregulation of MnSOD or complementary antioxidants is crucial to minimize mitochondrial dysfunction, preserve joint tissue integrity, and potentially mitigate the severity of AMI.113
Recent interest has centered on procyanidins, polyphenolic compounds found in foods such as antioxidants with anti-inflammatory properties.114 Procyanidins can directly scavenge free radicals, inhibit NF-κB activation, and boost endogenous antioxidant pathways, including MnSOD and glutathione peroxidase, thereby limiting oxidative damage to joint structures.115 By reducing the release of pro-inflammatory cytokines and diminishing ROS levels, these bioactive polyphenols may help alleviate the catabolic environment and the nociceptive drive that perpetuates spinal inhibitory reflexes.116 In doing so, procyanidins could indirectly aid in preserving muscle function and countering the progression of AMI.117
Supraspinal regions are not spared from the wave of inflammatory mediators.118 IL-6 and other cytokines that breach the blood-brain barrier or signal through circumventricular organs can modulate motor circuits within the cortex and subcortical structures, such as the basal ganglia and thalamus.119 Functional neuroimaging reveals that the secondary somatosensory cortex (SII) and pre-supplementary motor area (Pre-SMA) exhibit increased activation in individuals recovering from ACL injuries, reflecting compensatory strategies when proprioceptive feedback from mechanoreceptors is diminished.120 At the molecular level, the same intracellular pathways (e.g., Ca²⁺-dependent activation of calmodulin kinases, extracellular signal-regulated kinase [ERK] cascades, and downstream transcription factors like cAMP response element-binding protein [CREB]) that drive synaptic plasticity in pain circuits also shape cortical remodeling.121 Elevated or dysregulated production of neurotrophic factors—such as brain-derived neurotrophic factor (BDNF) or nerve growth factor (NGF)—can consolidate maladaptive sensorimotor patterns, leading to prolonged quadriceps inhibition, abnormal co-contractions, and joint stiffening that diminish functional recovery.122
The joint’s cytokine milieu eventually shifts toward an anti-inflammatory profile as IL-10 and IL-4 levels rise, dampening NF-κB-mediated gene transcription and promoting macrophage polarization to the M2 phenotype.123 Transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF) secreted by these M2 macrophages support tissue repair, collagen synthesis, and angiogenesis, guiding the joint toward structural and functional restoration.124 Nevertheless, if myostatin remains elevated, muscle regrowth is hampered, and if MnSOD activity is not sufficiently upregulated, unchecked oxidative stress can perpetuate tissue damage and maladaptive neurophysiological responses.125 Procyanidins or other dietary antioxidants could help compensate for the elevated ROS levels by supporting intrinsic antioxidant systems and curbing the chronic inflammatory drive.126 Meanwhile, if the CNS’s plastic changes—particularly central sensitization and enhanced inhibitory output on alpha motor neurons—remain unchecked, AMI may persist despite an improving local tissue environment.127
Interventions that address both the local inflammatory and broader neuroplastic changes are critical for restoring normal motor control.128 Pharmacologically, selective blockade of IL-1 or IL-6 can reduce catabolism and pain signaling, but care must be taken to avoid impairing tissue repair.129 Myostatin inhibitors could potentially assist in maintaining muscle mass, especially when combined with progressive exercise strategies that counteract the reflex-driven disuse of periarticular muscles.130 Enhancing MnSOD expression or activity, possibly via polyphenol-rich supplementation (including procyanidins), could bolster endogenous defense mechanisms against oxidative damage.131
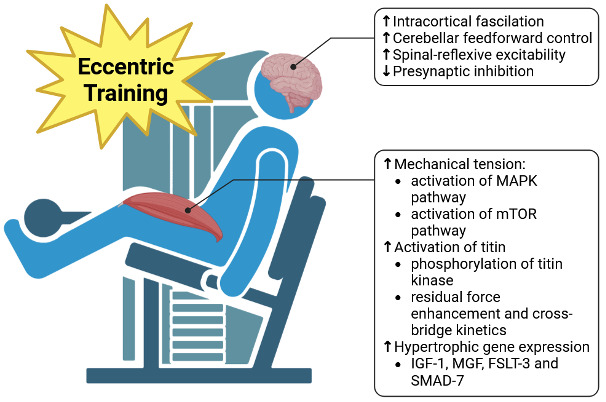
Neuromuscular electrical stimulation (NMES) and targeted exercise modalities, particularly eccentric and isometric exercises, have gained attention for their ability to disrupt AMI by re-engaging muscle fibers and recalibrating descending motor pathways.132 NMES recruits motor units in a non-selective pattern, often bypassing the inhibitory reflex arcs that hinder voluntary activation.133 This can promote muscle hypertrophy and maintain or even improve neuromuscular connectivity during periods when voluntary activation is suppressed.134 Eccentric exercise, by imposing controlled lengthening contractions, helps increase cross-bridge engagement while potentially enhancing titin-actin interactions that support residual force enhancement.135 These adaptations can counter the catabolic signals (including myostatin) and strengthen muscle fibers against disuse atrophy.136 Isometric exercises, characterized by force application without a change in muscle length, can provide early-stage loading that stabilizes the joint, facilitates neural drive, and mitigates further muscle wasting.137
By disrupting feedforward loops of cytokine-driven excitability, regulating myostatin’s atrophic signals, balancing ROS generation with MnSOD-mediated detoxification, and reactivating muscle fibers through NMES and specific exercise regimens, rehabilitation strategies can normalize sensorimotor function, reduce AMI, and protect against the long-term consequences of persistent quadriceps weakness and joint instability (Table 1).138 Through these multifaceted approaches—encompassing molecular, cellular, and biomechanical interventions—clinicians can expedite functional recovery and minimize the risk of re-injury following ACL trauma.139
4. Biomechanics of ACL injury
The biomechanics of an anterior cruciate ligament (ACL) injury are rooted in the complex interaction between joint anatomy, mechanical forces, and neuromuscular control, often occurring within a very narrow time frame during high-risk movements.141 The primary mechanisms leading to an ACL rupture involve excessive anterior tibial translation, internal tibial rotation, and valgus collapse at the knee joint.142 These forces create a multi-planar loading environment that overwhelms the structural capacity of the ACL to maintain knee stability, resulting in its failure.143
A key factor in ACL injury is the sagittal plane mechanics of the knee, particularly the generation of anterior tibial shear forces.144 When the knee is near full extension, the quadriceps muscle group exerts a significant anterior shear force on the tibia due to its line of pull.145 This force is especially pronounced during activities that involve sudden deceleration or landing from a jump, where the quadriceps contract forcefully to control knee flexion.146 The ground reaction forces during these movements further amplify the anterior shear by pushing the tibia forward relative to the femur.147 The ACL serves as the primary restraint against this anterior translation, and if the force exceeds the ligament’s tensile strength, it can lead to a rupturę.148
Internal tibial rotation is another critical component in the biomechanics of ACL injury.149 During dynamic movements such as cutting or pivoting, the foot is planted while the body changes direction, causing the tibia to rotate internally relative to the femur.150 The ACL resists this rotational force, but when combined with anterior shear, the stress on the ligament increases significantly.151 This combination places the ACL fibers under a helical load, making them more susceptible to mechanical failure.152 The degree of internal rotation is often influenced by factors such as muscle strength imbalances, particularly weakness in the hip external rotators and abductors, which fail to control excessive femoral internal rotation and adduction.153
Valgus collapse of the knee, characterized by the inward buckling of the knee joint, is a common mechanism contributing to ACL injuries.154 This occurs when there is excessive hip adduction and internal rotation, leading to a misalignment where the knee moves medially relative to the hip and ankle.155 Weakness in the hip musculature, especially the gluteus medius and maximus, compromises the ability to stabilize the pelvis and control femoral motion. As a result, the knee experiences increased valgus stress.156 When valgus collapse occurs simultaneously with internal tibial rotation and anterior shear forces, the ACL is subjected to a tri-planar load that greatly exceeds its structural capacity.157
Neuromuscular control plays a pivotal role in modulating these biomechanical forces.158 Adequate muscle activation patterns are essential for maintaining knee stability during high-risk movements.159 The hamstring muscles act as dynamic stabilizers by exerting a posterior force on the tibia, counteracting the anterior shear produced by the quadriceps.160 If hamstring activation is delayed or insufficient, the protective effect is lost, and the ACL bears a greater load.161 Similarly, proper activation of the hip muscles helps control femoral motion and prevents excessive valgus and rotational stresses on the knee.162
Fatigue can exacerbate neuromuscular deficits, diminishing the muscles’ ability to respond effectively to dynamic demands.163 As athletes become fatigued, their movement patterns often change, with decreased knee flexion angles during landing and reduced muscle activation levels.164 These alterations increase reliance on passive structures like the ACL for joint stability.165 Additionally, impaired proprioception following fatigue can delay reflexive muscle responses, further compromising knee stability.166
Anatomical factors also contribute to the biomechanics of ACL injury.167 A steep posterior tibial slope increases the tendency for the tibia to translate anteriorly under axial loads.168 This anatomical variation means that during weight-bearing activities, the tibia naturally slides forward relative to the femur, placing additional strain on the ACL.169 A narrower intercondylar notch of the femur can impinge on the ACL during knee extension and rotation, increasing the risk of injury.170 Females, on average, have a narrower notch width and a greater posterior tibial slope than males, which may partially explain the higher incidence of ACL injuries in female athletes.171
The timing of force application is crucial in ACL injury mechanisms.172 The injurious event typically occurs within the first 50 milliseconds after foot contact during dynamic movements like landing or cutting.173 This rapid loading does not allow sufficient time for protective neuromuscular responses, such as muscle co-contraction or reflexive adjustments, to mitigate the forces transmitted to the ACL.174 Consequently, the ligament experiences high-magnitude forces in a very short period, overwhelming its ability to maintain structural integrity.175
Biomechanical studies using motion analysis and force plate data have shown that individuals who sustain ACL injuries often exhibit specific movement patterns.176 These include landing with less knee flexion (stiff landing), increased quadriceps activation relative to hamstrings (quadriceps dominance), and greater knee valgus angles.177 These patterns result in higher anterior shear forces, increased valgus moments, and elevated internal rotation torques at the knee joint.178 Interventions aimed at modifying these movement patterns through neuromuscular training have been shown to reduce ACL injury risk by improving muscle activation timing, enhancing proprioception, and promoting safer landing and cutting techniques.179
In summary, the biomechanics of an ACL injury involve a complex interplay of excessive anterior tibial translation, internal tibial rotation, and valgus collapse, often exacerbated by neuromuscular control deficits and anatomical predispositions.180 These forces converge during high-risk movements, rapidly overloading the ACL fibers beyond their tensile capacity.181 Understanding these biomechanical factors is essential for developing effective prevention and rehabilitation strategies. Such strategies should focus on improving neuromuscular control, correcting faulty movement patterns, strengthening key muscle groups, and addressing individual anatomical risk factors to reduce the likelihood of ligament rupturę.182
5. Biomechanical principles in ACL injury prevention
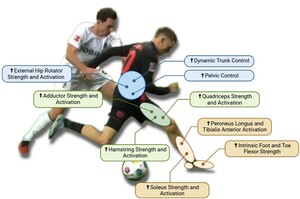
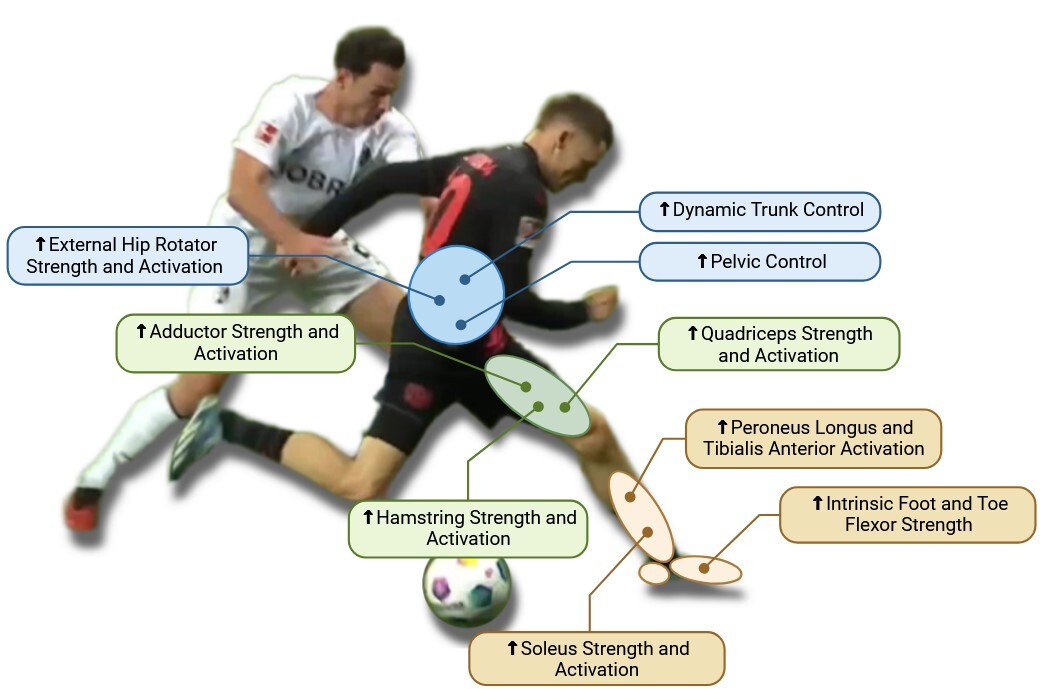
The biomechanical principles of ACL injury prevention focus on enhancing dynamic stability, optimizing joint alignment, and strengthening key muscle groups that control lower extremity movements.183 Central to these principles is the control of trunk movement, as the trunk comprises approximately half of the body’s total mass and must be effectively stabilized, especially during changes of direction.184 Poor trunk control leads to excessive lateral bending, increasing the moment arm in the frontal plane and redirecting the ground reaction force (GRF) vector, which places greater stress on the knee joint.185 In contrast, a medial trunk tilt is associated with faster and more efficient movement patterns because it shifts the center of mass closer to the knee, mitigating excessive joint loading.186 However, an excessive anterior trunk lean can elevate tensile strain on the hamstrings, increasing their susceptibility to injury.187 Therefore, training athletes to maintain appropriate trunk alignment is essential for optimal force distribution and knee protection (Figure 4).
The pelvis plays a crucial role in lower body stability, as pelvic tilt directly influences the alignment and orientation of the trunk during dynamic movements.188 Proper management of pelvic tilt allows an athlete to maintain a more vertical trunk position, aligning the center of mass with the intended direction of motion and reducing unnecessary compensatory forces at the knee.189 Hip control is another essential aspect, particularly through the activation of external rotators and gluteal muscles.190 These muscles help decrease knee abduction angles and minimize frontal plane knee moments, which is critical because increased valgus angles are strongly associated with ACL injury risk.191 Even small changes in valgus alignment can lead to significant increases in knee adduction moments, further stressing the ligament.192 Strong gluteal muscles, especially the gluteus medius and maximus, are necessary to resist these potentially injurious forces and prevent excessive valgus and internal rotation of the knee, hallmark risk factors for ACL tears.193
Similarly, the hamstring muscles are vital for ACL protection as they act as dynamic stabilizers, counteracting anterior tibial shear forces created by the quadriceps.194 This role is particularly important during deceleration and landing phases, where the hamstrings work to control knee flexion and reduce anterior tibial displacement, effectively offloading the ACL.195 A deficiency in hamstring strength, especially in the medial fibers like the semitendinosus, can result in poor knee control and increased reliance on quadriceps-dominant strategies, which amplify ACL strain.196 Proper hamstring conditioning should emphasize both strength and neuromuscular control, ensuring balanced activation between the medial and lateral hamstring components to promote symmetrical knee stabilization.197
The quadriceps are equally important, serving as the primary force generators for deceleration and propulsion.198 However, when quadriceps strength is not balanced with adequate hamstring strength, particularly during eccentric contractions, the resulting anterior shear force can be detrimental to the ACL.199 A weak quadriceps muscle group may lead to suboptimal braking strategies characterized by excessive trunk and hip flexion—a hip-dominant strategy—instead of controlled knee flexion.200 This compensatory pattern increases the eccentric loading on the hamstrings and heightens the risk of muscle strain or joint instability.201 Rehabilitation and prevention programs should therefore focus on enhancing eccentric quadriceps strength to support knee flexion and reduce detrimental shear forces.202
The soleus muscle, often overlooked, plays a pivotal role in ACL biomechanics by creating a posterior shear force that counters anterior tibial translation, providing critical support during braking and propulsion.203 Strengthening the soleus is essential, especially for athletes who frequently perform high-impact movements, as it stabilizes the tibia and prevents forward sliding that could stress the ACL.204 Additionally, the activation of the peroneus longus and tibialis anterior muscles is integral to ankle stability, ensuring that the ankle joint remains aligned and capable of resisting excessive inversion, supination, and internal rotation.205 These ankle mechanics are crucial because instability at the ankle can cascade into poor knee mechanics, increasing the risk of ACL injury.206
The intrinsic muscles of the foot also significantly contribute to lower limb biomechanics by supporting the medial longitudinal arch and enabling effective energy absorption and return.207 A strong, stable foot arch helps maintain proper alignment of the kinetic chain, reducing compensatory movements that may propagate up to the knee and hip.208 This stable foundation is essential for athletes performing repetitive, high-load movements, as it ensures that forces are efficiently transmitted and absorbed, preventing overload at proximal joints.209
Adductor strength is another key factor in ACL injury prevention, particularly during the push-off phase of directional changes.210 In this phase, when the hip and knee are extended and the pelvis is rotating, the adductor muscles—especially the adductor longus and gracilis—experience high elongation speeds and are subjected to eccentric loading.211 Insufficient adductor strength can increase the risk of muscle strain or groin injuries, which may alter gait mechanics and predispose the athlete to compensatory knee loading patterns that elevate ACL injury risk.212 Strengthening these muscles enhances hip stability and improves the ability to generate powerful lateral movements, reducing the risk of knee joint collapse during rapid direction changes.213
Overall, ACL injury prevention should focus on a comprehensive approach that includes stabilization and coordination of proximal segments like the trunk and pelvis, as well as targeted strengthening and activation of distal muscle groups such as the quadriceps, hamstrings, and ankle stabilizers.214 By optimizing these biomechanical elements, athletes can achieve better control over knee alignment and movement patterns, reducing the likelihood of high-risk positions and minimizing the forces that contribute to ACL injuries. Integrating proprioceptive training, neuromuscular re-education, and strength conditioning in these key muscle groups will help create a resilient and well-coordinated kinetic chain that effectively protects the ACL from excessive multidirectional loading (Table 2).215
6. Neuromechanics of ACL injury
The neuromechanics of Anterior Cruciate Ligament (ACL) injury encompass a complex interplay between the nervous system, musculoskeletal structures, and biomechanical forces that collectively create conditions where the ACL is subjected to excessive stress, ultimately leading to its failure.216 Understanding these neuromechanical factors is crucial because the risk of ACL injury is not solely due to anatomical or mechanical predispositions; it also results from impaired neuromuscular control, altered reflex responses, and suboptimal muscle coordination.217 The ACL functions not only as a passive stabilizer, preventing excessive anterior translation and rotation of the tibia, but also serves as a critical proprioceptive organ.218 It provides the central nervous system (CNS) with essential information about knee joint position and loading.219 When these neuromuscular control systems are disrupted—whether due to fatigue, cognitive load, or previous injury—there is a breakdown in the brain’s ability to effectively coordinate movements, increasing the likelihood of injury.220
One of the primary neuromechanical risk factors for ACL injury is altered proprioception and delayed neuromuscular responses.221 The ACL is richly innervated with mechanoreceptors, including Ruffini endings and Pacinian corpuscles, which detect joint tension, speed of movement, and positional changes.222 These receptors transmit proprioceptive information to the CNS, where it is integrated with visual and vestibular inputs to coordinate muscular responses.223 When an athlete performs a high-risk movement, such as sudden deceleration or pivoting, the proprioceptive feedback from the ACL plays a critical role in initiating reflexive muscle contractions that stabilize the knee.224 If this proprioceptive feedback is altered—due to fatigue, previous joint instability, or a lack of neuromuscular conditioning—the timing and magnitude of muscle contractions may be insufficient to counteract destabilizing forces.225 This delay in muscular activation increases the window of time during which the ACL is vulnerable to excessive loading, making it more susceptible to rupturę.226
Another key element in the neuromechanics of ACL injury is the role of altered feedforward and feedback motor control mechanisms.227 Feedforward control refers to the CNS’s anticipatory activation of muscles based on expected movement patterns and environmental conditions.228 For instance, when an athlete anticipates landing from a jump, the CNS pre-activates the hamstrings and quadriceps to stabilize the knee joint before ground contact.229 This pre-activation is critical for stiffening the joint and distributing forces evenly through the musculature and ligaments.230 However, if there is a disruption in feedforward control—such as in conditions of fatigue or cognitive distraction—the timing and coordination of these muscle contractions are compromised, leading to an inadequate muscular response that fails to protect the ACL.231
Similarly, feedback mechanisms involve reflexive adjustments to unexpected perturbations and are essential for maintaining dynamic knee stability.232 When the knee is subjected to sudden external forces, such as a rapid change in direction or unanticipated ground contact, the CNS relies on rapid reflex loops to activate stabilizing muscles like the hamstrings and gastrocnemius to counteract anterior tibial translation and internal rotation.233–235 Studies have shown that these reflexive responses are delayed and attenuated in athletes with poor neuromuscular conditioning or in those recovering from previous ACL injuries. This neuromuscular delay reduces the effectiveness of the muscular response, allowing potentially injurious forces to be transmitted directly to the ACL before the muscles have time to react.236
Furthermore, the neuromechanics of ACL injury are influenced by the interplay between central motor control strategies and peripheral muscle activation patterns.237 The brain’s motor cortex, supplementary motor areas, and cerebellum are responsible for generating motor commands and coordinating complex movement sequences.238 When athletes are fatigued or under high cognitive load, there is increased reliance on higher-order motor areas to consciously control movements that would typically be automatic.239 This increased cortical involvement can lead to a phenomenon known as “cortical over-recruitment,” where the brain compensates for reduced proprioceptive feedback by increasing conscious attention to knee control.240 While this strategy may temporarily enhance stability, it comes at the cost of slower reaction times, reduced movement fluidity, and a higher incidence of errors during high-speed or unpredictable maneuvers.241 The result is a greater likelihood of the knee entering a high-risk position, such as excessive valgus or internal rotation, which significantly increases ACL strain.242
The influence of fatigue on neuromechanical control is another critical factor. Fatigue not only affects muscle strength and endurance but also impairs the CNS’s ability to accurately process sensory information and coordinate motor responses.243 When muscles are fatigued, their capacity to generate force rapidly decreases, and their ability to stabilize the joint becomes compromised.244 Additionally, fatigue disrupts proprioceptive acuity, reducing the athlete’s awareness of joint position and increasing reliance on visual and vestibular inputs.245 This shift in sensory dominance alters motor strategies, leading to a delayed or exaggerated muscular response that fails to protect the ACL during high-stress movements.246 The combination of reduced muscle force production, impaired reflexes, and altered sensory integration creates a scenario where the knee joint is highly vulnerable to injury, even during routine athletic tasks.247–249
Muscle activation imbalances and altered co-contraction patterns are also prominent neuromechanical contributors to ACL injury.250 Optimal knee stability is achieved through a delicate balance between agonist and antagonist muscle groups, primarily the quadriceps and hamstrings.251 The quadriceps produce a strong anterior shear force that pushes the tibia forward relative to the femur, while the hamstrings generate a posterior shear force that counteracts this movement.252,253 In athletes with weak or delayed hamstring activation, there is an over-reliance on the quadriceps to control knee stability, which significantly increases anterior tibial translation and ACL loading.254 This imbalance is particularly problematic during high-risk movements like sudden deceleration or landing, where the quadriceps are heavily activated, and the hamstrings may not engage quickly enough to counteract the anterior shear forces.255 The resulting high anterior tibial translation places the ACL under excessive strain, leading to its rupturę.256
Moreover, altered lower limb kinematics, such as increased knee valgus or internal rotation, are often the result of poor neuromuscular control at the hip and trunk.257 The gluteal muscles, particularly the gluteus medius, are responsible for maintaining frontal plane stability of the pelvis and femur.258 When these muscles are weak or poorly coordinated, the femur tends to collapse medially during dynamic tasks, resulting in increased knee valgus and internal tibial rotation.259 This alignment significantly increases ACL strain, as the ligament must resist both rotational and valgus forces.260 Training programs aimed at enhancing hip and core stability can significantly reduce this risk by improving the alignment and control of the lower extremity, thereby decreasing the mechanical load on the ACL.261
In conclusion, the neuromechanics of ACL injury involve a multifaceted interplay of proprioceptive feedback, neuromuscular control, motor planning, and reflex responses, all of which contribute to the dynamic stability of the knee joint.262 Disruptions in any of these systems—whether due to fatigue, previous injury, poor training, or cognitive load—can lead to altered motor strategies that increase the likelihood of the knee entering a high-risk position.263 Prevention strategies should therefore focus not only on strengthening key muscle groups but also on enhancing neuromuscular coordination, reflex timing, and proprioceptive accuracy. By targeting these neuromechanical factors, it is possible to reduce the incidence of ACL injuries and improve overall knee joint health and performance.264
7. Sensorimotor System Neuroanatomy
The neurophysiological aspects of ACL injury involve an intricate understanding of the sensorimotor system’s neuroanatomy, which is crucial for comprehending how the central and peripheral nervous systems interact with musculoskeletal structures to regulate movement, maintain joint stability, and respond to external perturbations.265 The sensorimotor system encompasses a complex network of sensory receptors, peripheral nerves, spinal cord circuits, and multiple brain regions that work together to integrate sensory inputs and generate appropriate motor outputs.266 This sophisticated coordination allows for fine-tuned control of voluntary movements and rapid initiation of reflexive responses essential for maintaining balance, posture, and dynamic joint stability.267
At the peripheral level, specialized sensory receptors embedded within muscles, tendons, ligaments, and joint capsules initiate the sensorimotor pathway.268 Muscle spindles, located within muscle fibers, are sensitive to changes in muscle length and the rate of stretch, providing real-time information about muscle dynamics.269 They play a pivotal role in regulating muscle tone and facilitating the stretch reflex, which helps maintain posture and respond to sudden changes in muscle length. Golgi tendon organs, situated at the junction between muscles and tendons, monitor changes in muscle tension and force production.270 They are critical for preventing muscle damage by initiating inhibitory responses when excessive force is detected, thus modulating muscle contractions to safeguard the musculoskeletal system.271
Ligamentous mechanoreceptors, such as Ruffini endings, Pacinian corpuscles, and Golgi-like receptors, are densely distributed within the ACL and other major knee ligaments.272 Ruffini endings detect sustained pressure and stretch, providing information about joint position and movement direction.273 Pacinian corpuscles respond to rapid changes in pressure and high-frequency vibration, detecting dynamic joint movements.274 Golgi-like receptors sense tension within the ligaments, informing the central nervous system (CNS) about the degree of stretch and load on joint structures.275 Collectively, these mechanoreceptors form the first line of sensory defense against mechanical instability, continuously monitoring joint dynamics and providing essential feedback necessary for regulating knee stability.276
The afferent signals generated by these peripheral receptors travel along myelinated sensory nerve fibers and enter the dorsal horn of the spinal cord.277 Here, they synapse onto interneurons and ascending projection neurons. Some of these signals are integrated within the spinal cord to form reflex arcs, enabling rapid, involuntary responses to sudden stimuli.278 For example, the monosynaptic stretch reflex involves a direct connection between sensory afferents and motor efferents, allowing immediate muscle contraction in response to muscle stretch, which is crucial for maintaining joint stability during unexpected perturbations.279
Ascending pathways transmit sensory information to higher brain centers for further processing.280 The dorsal column-medial lemniscal pathway carries fine touch and proprioceptive information to the brainstem and then to the thalamus, which acts as a relay station.281 The thalamus filters and organizes sensory inputs before transmitting them to the somatosensory cortex in the parietal lobe.282 The primary somatosensory cortex processes detailed information about joint position, movement, and tactile sensations, contributing to the conscious perception of proprioception.283 This cortical processing enables the integration of sensory inputs with cognitive functions, such as attention and planning, allowing for conscious modulation of movements and adjustments in response to environmental demands.284
Motor responses are orchestrated by the motor cortex, including the primary motor cortex, premotor cortex, and supplementary motor area.285 The primary motor cortex is responsible for initiating voluntary muscle contractions by sending descending motor commands through the corticospinal tract to motor neurons in the spinal cord.286 The premotor cortex and supplementary motor area are involved in planning and coordinating complex movements, integrating sensory information and preparing the motor system for action.287 These areas are essential for tasks that require coordination, timing, and sequencing of movements, such as those involved in athletic activities posing a risk for ACL injury.288
The cerebellum plays a crucial role in motor control by integrating sensory inputs from the proprioceptive system with motor commands from the cerebral cortex.289 It is responsible for fine-tuning movements, ensuring accuracy, coordination, and balance.290 The cerebellum adjusts motor output based on sensory feedback, allowing for smooth and precise execution of movements.291 It is also involved in motor learning, adapting motor programs through practice and experience.292 Dysfunction in cerebellar processing can lead to impaired coordination, increasing the risk of injury due to unsteady or inaccurate movements.293
Basal ganglia, a group of subcortical nuclei, are involved in motor control, particularly in the initiation and regulation of voluntary movements.294 They modulate motor commands to prevent unwanted movements and ensure smooth transitions between movement sequences.295 The basal ganglia receive inputs from the cerebral cortex and send outputs back via the thalamus, forming circuits essential for motor planning and execution.296 Disruptions in basal ganglia function can result in movement disorders characterized by rigidity or involuntary movements, potentially affecting an individual’s ability to perform complex motor tasks safely.297
Integration of sensory and motor information also involves the parietal and frontal association cortices.298 The posterior parietal cortex integrates multisensory information, contributing to spatial awareness and perception of body position in space.299 This region is essential for guiding movements based on sensory inputs, such as reaching or navigating through an environment.300 The prefrontal cortex is involved in higher-order cognitive functions, including decision-making, attention, and executive control. It plays a role in selecting appropriate motor responses based on contextual cues and anticipated outcomes.301 Cognitive load and attentional demands can influence motor performance by affecting these cortical areas, potentially leading to decreased movement accuracy and increased injury risk under conditions of mental fatigue or distraction.302
Descending motor pathways, such as the corticospinal tract, transmit motor commands from the cortex to the spinal cord, where they synapse onto motor neurons that innervate skeletal muscles.303 Other descending tracts, like the reticulospinal and vestibulospinal tracts, originate from brainstem nuclei and contribute to the regulation of muscle tone, posture, and reflexes.304 These pathways are important for maintaining balance and adjusting body position in response to sensory feedback, particularly during dynamic activities that challenge stability.305
The spinal cord serves as a crucial hub for integrating sensory inputs and generating motor outputs.306 Interneurons within the spinal cord facilitate complex reflex circuits that coordinate muscle activity across different joints and muscle groups.307 For example, the flexor withdrawal reflex involves activation of flexor muscles and inhibition of extensor muscles in response to painful stimuli, allowing rapid withdrawal from harm.308 Such reflexes are essential protective mechanisms that operate without conscious control, providing immediate responses to potential threats.309
Neuromuscular junctions, where motor neurons synapse onto muscle fibers, are critical sites for translating neural signals into mechanical actions.310 The release of neurotransmitters like acetylcholine triggers muscle contraction by initiating electrical changes in the muscle membrane, leading to the sliding of actin and myosin filaments.311 Efficiency of neuromuscular transmission can be affected by factors such as fatigue, neuromuscular diseases, or pharmacological agents, impacting muscle performance and coordination.312
Understanding the neurophysiological mechanisms underlying sensorimotor control has significant implications for ACL injury prevention and rehabilitation.313 Enhancing proprioceptive training can improve the sensitivity of sensory receptors and efficiency of neural pathways involved in movement control.314 Balance and coordination exercises strengthen the integration between sensory inputs and motor outputs, leading to more precise and stable movements.315 Cognitive training that reduces the impact of mental fatigue and improves attentional focus can mitigate effects of cognitive load on motor performance.316
Injury or degeneration of any components within this sensorimotor network can disrupt the delicate balance required for optimal movement control.317 Peripheral nerve injuries can impair sensory feedback, leading to delayed or inappropriate motor responses.318 Spinal cord injuries can interrupt ascending and descending pathways, resulting in loss of sensation or motor function below the level of the lesion.319 Central nervous system disorders affecting brain regions involved in motor control can alter movement planning and execution, increasing the risk of injury during physical activities.320
In conclusion, the neuroanatomy and neurophysiology of the sensorimotor system involve a highly coordinated and complex network that integrates sensory information and generates precise motor outputs necessary for movement regulation, joint stability, and response to external perturbations.321 Disruptions in any part of this system—whether due to injury, fatigue, or cognitive factors—can compromise neuromuscular control and increase the risk of ACL injuries.322 A comprehensive understanding of these neurophysiological mechanisms is essential for developing effective training, prevention, and rehabilitation strategies aimed at enhancing sensorimotor function and reducing injury risk.
8. ACL injury is an Sensorimotor System Error
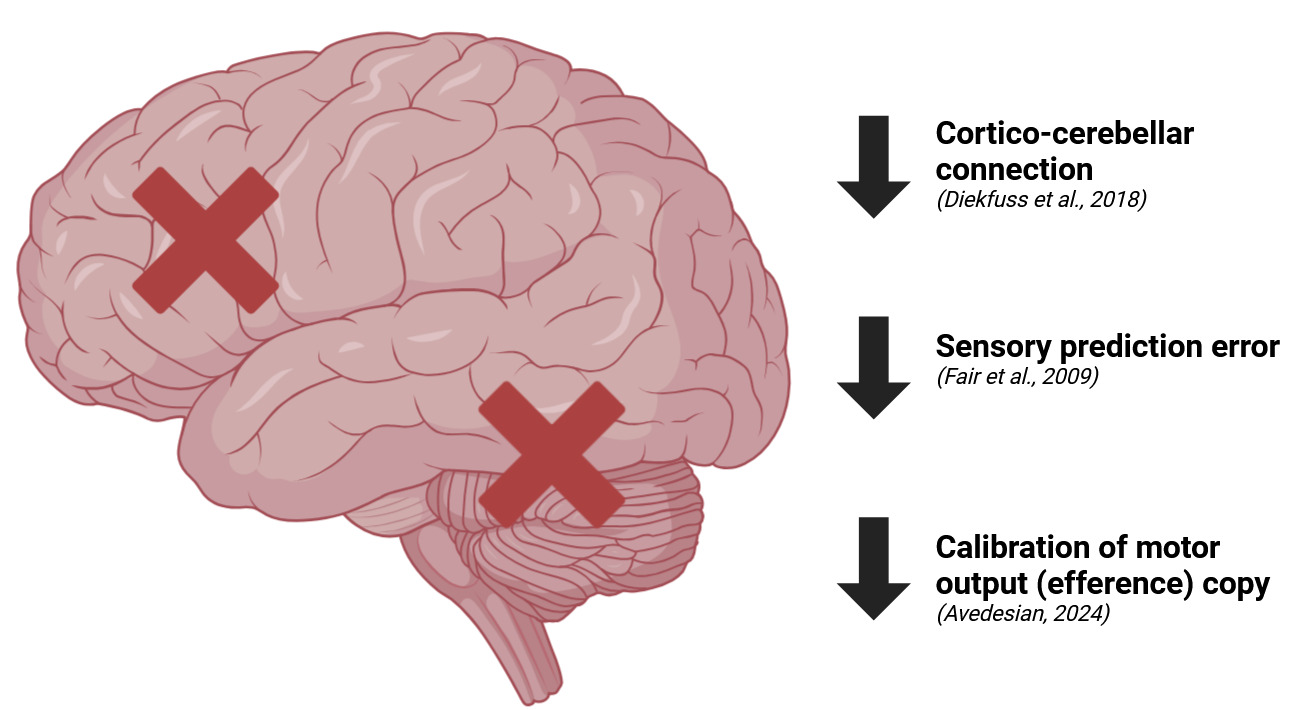
The neurophysiological aspects of anterior cruciate ligament (ACL) injury highlight how a breakdown in the sensorimotor system contributes to an increased risk of ligament damage.323,324 An ACL injury can be understood as a sensorimotor system error, where failure occurs not only at the structural level of the ligament but also within the complex neural networks that integrate sensory feedback and motor control (Figure 5).140 The sensorimotor system is responsible for maintaining joint stability, coordinating precise movements, and responding appropriately to dynamic changes in the environment.325 When this system is disrupted, the capacity to predict, detect, and respond to mechanical stressors at the knee joint becomes impaired, leading to a higher likelihood of the joint entering a high-risk position that predisposes the ACL to excessive strain and eventual rupturę.326
One fundamental mechanism through which the sensorimotor system operates is the integration of feedforward and feedback control processes.327 Feedforward control involves the central nervous system’s (CNS) ability to anticipate and plan movements based on prior experience and internal models of body dynamics, allowing for proactive stabilization of joints and preparation for expected demands.328 Feedback control entails real-time adjustments of motor outputs in response to sensory feedback from proprioceptive, visual, and vestibular systems, enabling reactive corrections to unexpected perturbations.329 In the context of ACL injury, disruptions in both feedforward and feedback control mechanisms can compromise joint stability and increase the risk of injury.330
A critical component of sensorimotor control is the cerebellar-cortical connection, which plays a vital role in motor coordination, balance, and the fine-tuning of movement patterns.331 The cerebellum integrates sensory information to produce smooth, coordinated movements and adjusts for deviations from the intended motor plan.332 It contributes to feedforward control by generating anticipatory motor commands using internal models to predict the sensory consequences of movements.333 This allows for preemptive muscle activation patterns that stabilize the knee joint before potentially injurious movements occur.334 In feedback control, the cerebellum processes sensory feedback to detect discrepancies between expected and actual outcomes, facilitating rapid adjustments to motor commands.335 Disruptions in cerebellar-cortical connectivity impair the CNS’s ability to modulate motor commands based on sensory feedback, leading to less accurate and less stable joint control.336 The cerebellum’s role in detecting sensory prediction error—a discrepancy between predicted and actual sensory feedback—is crucial for preventing abnormal knee kinematics that can strain the ACL.337 A weakened cerebellar-cortical loop results in diminished error detection and correction, making it more difficult for the brain to adapt movement strategies in response to changing environmental demands.338
Sensory prediction error is fundamental to understanding how the sensorimotor system operates under normal conditions and how it fails when neuromuscular deficits are present.339 It refers to the discrepancy between the predicted sensory outcome of a movement and the actual sensory feedback received during or after the movement.340 When this error is detected, the cerebellum updates and refines motor commands to minimize future discrepancies, optimizing movement precision.341 In individuals with ACL injuries or impaired neuromuscular function, there is evidence of a reduced ability to detect and correct sensory prediction errors.342 Impairments in feedforward control lead to inadequate anticipatory muscle activation, such as insufficient pre-activation of the hamstrings and quadriceps before ground contact during landing.343 This lack of preparatory muscle activity compromises joint stiffness and increases reliance on feedback mechanisms to correct for joint instability.344 If feedback control is also compromised due to delayed or diminished reflex responses, the knee joint becomes vulnerable to excessive loading before corrective muscle activations can occur.345 This impaired prediction results in greater reliance on reactive motor strategies rather than anticipatory control, which are inherently slower and less effective at protecting the knee from rapid, high-impact forces.346
Another critical factor in the sensorimotor system’s failure during ACL injury is the disruption of the efference copy mechanism.347 Efference copy refers to the internal copy of the motor command sent to sensory regions of the brain simultaneously with the execution of a movement.348 This mechanism allows the CNS to predict the sensory consequences of an action and differentiate between self-generated and externally generated movements.349 Following an ACL injury, the calibration of the efference copy can be disrupted, meaning the motor system’s predictions about joint position and movement outcomes become less accurate.350 This inaccuracy impairs feedforward control, diminishing the CNS’s ability to anticipate and prepare for movement demands. Consequently, there is a decreased capacity for anticipatory adjustments, necessitating greater reliance on feedback control to maintain joint stability.351 However, during high-speed athletic movements, the latency of feedback responses may be insufficient to prevent injury, underscoring the importance of intact feedforward mechanisms.352 The mismatch between expected and actual sensory feedback compromises the ability to generate appropriate muscle activations to stabilize the knee. Without a correctly calibrated efference copy, the CNS cannot effectively pre-activate the muscles surrounding the knee to counteract potentially injurious forces, leaving the ACL vulnerable during high-risk movements.
The breakdown in efference copy and sensory prediction error is further complicated by deficits in sensory integration within the sensorimotor network.353 Key regions responsible for sensory integration, such as the parietal cortex and supplementary motor area, may receive less reliable proprioceptive input from the injured knee. This altered sensory input disrupts the CNS’s internal representation of the knee joint, known as the body schema, which is essential for planning and executing movements. Impaired sensory integration affects both feedforward and feedback control processes.354 In feedforward control, inaccurate internal representations lead to flawed anticipatory motor commands, resulting in inappropriate muscle activation patterns. In feedback control, unreliable sensory inputs hinder the CNS’s ability to detect and correct deviations from intended movement trajectories promptly, further compromising joint stability.355 When the body schema is inaccurate, the motor commands generated by the CNS are less precise, leading to joint positions and movement patterns that increase the strain on the ACL. This disruption is exacerbated by decreased cerebellar-cortical connectivity, as the cerebellum plays a pivotal role in refining sensory inputs and updating the body schema in real time.356
The inability to integrate and process sensory information efficiently leads to maladaptive motor control strategies. Individuals with ACL injuries often adopt compensatory movement patterns, such as increased co-contraction of the quadriceps and hamstrings, to stabilize the knee.357 While these strategies may temporarily reduce knee laxity, they come at the cost of increased joint stiffness and reduced dynamic stability.358 The shift from feedforward to feedback-dominant control increases dependency on reflexive responses that may not be sufficiently rapid or coordinated to prevent injury during dynamic activities. Excessive reliance on feedback control can lead to delayed muscle activations, allowing harmful joint positions to be reached before corrective actions occur.359 This stiffening response, driven by over-reliance on spinal-level reflexes rather than cortical control, reduces the knee’s ability to absorb and dissipate forces effectively, potentially leading to excessive loading of the ACL during high-speed tasks like cutting, pivoting, or landing.360
Furthermore, the altered sensorimotor control following an ACL injury is not limited to the knee joint but extends to other parts of the kinetic chain, including the hip, ankle, and trunk.361 The brain’s altered representation of the knee joint affects its ability to coordinate proximal and distal segments effectively, leading to increased trunk sway, hip adduction, and foot instability. These maladaptive changes are further evidence of a global sensorimotor system error, where the entire neuromuscular network is disrupted, not just local joint mechanics.362 The over-reliance on conscious control and visual feedback indicates a breakdown in feedforward control mechanisms, where automatic anticipatory adjustments are replaced by slower, cognitively demanding processes.363 This reduces movement efficiency and impairs the timely initiation of protective muscle activations, increasing the likelihood of injury. This global disruption is reflected in altered activation patterns in the motor cortex, increased reliance on visual feedback, and a shift in motor control strategies from automatic to more conscious, effortful control. The increased cognitive load associated with conscious control of movement can further degrade performance and heighten the risk of secondary injuries.364
Additionally, long-term neuroplastic changes following an ACL injury contribute to persistent sensorimotor deficits. Decreased cerebellar-cortical connectivity is accompanied by changes in the structure and function of the sensorimotor cortex.365 Research has shown that the cortical representation of the injured knee becomes less defined, indicating a loss of proprioceptive acuity and motor control. These cortical changes are accompanied by alterations in subcortical structures such as the basal ganglia and thalamus, which are involved in coordinating complex movements and regulating movement initiation.366 The neuroplastic changes negatively impact both feedforward and feedback control. With altered cortical and subcortical processing, the CNS’s ability to generate accurate anticipatory motor commands is compromised, and the efficacy of reflexive responses is diminished.367 The reorganization of these neural networks leads to reliance on maladaptive motor patterns, such as increased knee valgus or delayed hamstring activation, perpetuating the risk of further injury.368
In conclusion, an ACL injury represents a sensorimotor system error characterized by disrupted sensory prediction, impaired motor output calibration, and altered neural connectivity.369 The decreased cerebellar-cortical connection impairs the CNS’s ability to integrate sensory feedback and adjust motor commands in real time, leading to a breakdown in joint stability.370 The diminished sensory prediction error and disrupted efference copy result in inaccurate motor planning and execution, making the knee more vulnerable to high-risk positions during dynamic movements. Addressing these neuromechanical deficits through targeted rehabilitation that focuses on restoring proprioceptive acuity, recalibrating motor output, and enhancing cerebellar-cortical connectivity is essential for reducing the risk of re-injury and optimizing long-term functional outcomes.371–373 Rehabilitation programs should incorporate training that enhances both feedforward and feedback control mechanisms, including exercises that improve anticipatory muscle activation patterns, such as plyometrics and sport-specific drills, and activities that enhance reflexive responses and sensory integration, like balance training and reactive agility tasks.374–376 By strengthening both control systems, individuals can regain the ability to predict, detect, and respond to mechanical stressors effectively, thereby reducing the likelihood of future ACL injuries.377
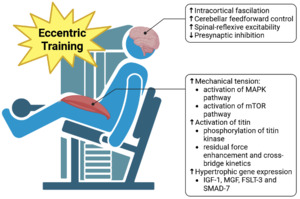
9. Molecular Basis for Intrinsic Muscle Properties. Preactivation, Cross-Bridging Kinetics, Residual Force Enhacement and Short-Range Stiffness
The intrinsic properties of muscles are deeply rooted in their molecular and biomechanical characteristics, which govern how they generate force, respond to external stimuli, and adapt to different functional demands (Figure 6).378 At the molecular level, these properties are primarily defined by the interactions of contractile proteins, regulatory mechanisms, and structural proteins within muscle fibers.379 These molecular interactions collectively influence the muscle’s ability to produce force, maintain stability, and dynamically respond to changes in load and movement.380 Key intrinsic properties such as preactivation, cross-bridge kinetics, and residual force enhancement each play distinct roles in muscle performance and neuromuscular control during complex motor tasks encountered in athletic and rehabilitative contexts.381
Muscle contraction begins at the sarcomeric level, where thin actin filaments slide over thick myosin filaments in a highly coordinated cycle.382 The structural alignment of these filaments is maintained by titin—which spans from the Z-disc to the M-line of the sarcomere—and by desmin, an intermediate filament protein.383 Desmin plays a crucial role in maintaining the spatial organization of myofibrils, ensuring that adjacent sarcomeres are aligned both longitudinally and laterally.384 By linking neighboring Z-discs, desmin helps distribute mechanical stress across the muscle fiber and synchronizes sarcomere contraction, contributing to efficient force transmission.385
In addition to desmin, muscle fibers possess specialized structures called costameres.386 Located at regular intervals along the sarcolemma, costameres anchor the contractile apparatus to the extracellular matrix (ECM) via integrins, dystroglycan complexes, and other adaptor proteins.387 This arrangement forms a mechanical link between the Z-discs of each sarcomere and the surrounding connective tissue.388 As a result, a portion of the contractile force generated by the myofibrils is transmitted laterally through the costameres to the ECM—rather than just longitudinally along the tendon-to-bone axis.389 This lateral force transmission mechanism stabilizes individual myofibrils during rapid contractions and sudden stretches, allowing muscles to withstand large loads while minimizing damage or deformation of the sarcolemma.390
At the cellular level, each muscle fiber (myofiber) contains multiple myofibrils, which are composed of repeated sarcomeres.391 Satellite cells—muscle stem cells located between the basal lamina and sarcolemma—play a key role in muscle repair and hypertrophy, influencing how muscles adapt over time.392 Hormones and growth factors such as insulin-like growth factor 1 (IGF-1), testosterone, and myostatin modulate satellite cell activation, proliferation, and differentiation, thereby affecting muscle mass and function.393 Through these adaptive processes, muscles can alter their cellular and molecular landscape to meet changing mechanical demands, whether from high-intensity exercise, chronic loading, or injury recovery.394
Preactivation is the anticipatory activation of muscles before an external load or movement occurs, priming the muscle fibers for subsequent force production.395 This process involves the early recruitment of motor units and the pre-setting of muscle stiffness, which are critical for stabilizing joints and optimizing force transmission during high-speed or high-impact movements.396 On a molecular level, preactivation is heavily influenced by the availability of intracellular calcium ions (Ca²⁺), the sensitivity of the contractile apparatus to calcium, and the phosphorylation state of regulatory proteins such as troponin and tropomyosin.397 When a motor neuron transmits an action potential to a muscle fiber, it triggers depolarization of the sarcolemma and the transverse (T) tubules, leading to the activation of voltage-sensitive dihydropyridine receptors (DHPRs).398 These receptors are mechanically coupled to ryanodine receptors (RyR1) on the sarcoplasmic reticulum membrane, causing them to release Ca²⁺ into the cytosol.399 The influx of Ca²⁺ binds to the troponin complex on the actin filaments, specifically troponin C (TnC), inducing a conformational change that shifts tropomyosin and exposes the myosin-binding sites on actin.400
During preactivation, increased calcium sensitivity—often mediated by post-translational modifications such as phosphorylation—enhances the readiness of muscle fibers to generate force quickly and efficiently.401 Phosphorylation of troponin I (TnI) and troponin T (TnT) by protein kinases can alter the calcium-binding properties of the troponin complex, effectively lowering the threshold for muscle activation.402 This heightened calcium sensitivity reduces the latency period between neural activation and force development, ensuring that the muscle can respond rapidly to sudden perturbations or shifts in load.403 Additionally, phosphorylation of myosin regulatory light chains (RLCs) by myosin light chain kinase (MLCK), which is activated by Ca²⁺-calmodulin complexes, increases the stiffness of the myosin head and enhances its ability to bind to actin, further contributing to rapid force generation.404
Preactivation also involves the recruitment of specific myosin heavy chain (MyHC) isoforms that possess distinct kinetic properties.405 Fast-twitch MyHC isoforms, such as MyHC IIa and IIx, are characterized by rapid cross-bridge cycling rates and high ATPase activity, allowing for quick and powerful contractions necessary for explosive movements.406 These isoforms have a higher rate of ATP hydrolysis, which fuels the rapid detachment and reattachment of the myosin head during cross-bridge cycling.407 In contrast, slow-twitch isoforms (e.g., MyHC I) exhibit slower cross-bridge kinetics but are more resistant to fatigue due to their greater reliance on oxidative metabolism and more efficient ATP utilization.408 The selective recruitment of these myosin isoforms during preactivation is modulated by motor unit firing patterns determined by the central nervous system, based on the anticipated demands of the task.409
The process of muscle contraction is governed by the cyclic interaction of myosin heads with actin filaments, known as cross-bridge cycling.410 This cycle involves a series of molecular events—attachment, power stroke, detachment, and reattachment—that convert chemical energy stored in ATP molecules into mechanical force.411 The kinetics of cross-bridge cycling determine how quickly and efficiently a muscle can generate force and adapt to changing mechanical demands.412 The rate of cross-bridge attachment and detachment is regulated by the intrinsic ATPase activity of the myosin head and the availability of ATP, which fuels the power stroke and subsequent release of the myosin head from actin.413
During a typical cross-bridge cycle, the myosin head starts in a low-energy state bound to actin in a rigor configuration.414 Binding of ATP to the myosin head reduces its affinity for actin, causing it to detach.415 The ATP is then hydrolyzed to ADP and inorganic phosphate (Pi), which energizes the myosin head and induces a conformational change to a “cocked” high-energy state.416 The myosin head then reattaches to a new position on the actin filament.417 Release of Pi initiates the power stroke, where the myosin head pivots and pulls the actin filament toward the center of the sarcomere, generating tension.418 ADP is subsequently released, and the myosin head remains tightly bound to actin until another ATP molecule binds, repeating the cycle.419 The speed and efficiency of these transitions—collectively referred to as cross-bridge kinetics—are critical for muscle performance, with faster kinetics resulting in greater power output at high shortening velocities.420
The kinetics of cross-bridge cycling are influenced by various molecular factors, including the isoform composition of myosin and the phosphorylation of myosin light chains.421 Phosphorylation of the regulatory light chains increases the stiffness of the myosin neck region, enhancing the coupling between the myosin head and the lever arm, which increases the force generated during the power stroke.422 This modification can also increase the probability of the myosin head binding to actin in a force-producing state, thereby enhancing overall force production.423 The structural arrangement of the sarcomeres and the elasticity of titin—a giant protein that spans from the Z-disc to the M-line of the sarcomere—also modulate cross-bridge kinetics by affecting passive tension and the stability of the actin-myosin interaction.424
Titin acts as a molecular spring that stores and releases elastic energy during muscle contractions, contributing to overall force output and resistance to stretch.425 It provides passive tension when the muscle is stretched, helping to maintain the alignment of thick and thin filaments within the sarcomere.426 Titin’s elasticity is regulated by its extensible regions, which can unfold under mechanical stress and refold when the stress is relieved, thereby contributing to the muscle’s viscoelastic properties.427 Post-translational modifications of titin, such as phosphorylation by kinases like protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CaMKII), can alter its stiffness and influence muscle mechanics.428
Costameres, in turn, help ensure that both active and passive forces generated within the sarcomere are effectively transferred to the surrounding muscle cell membrane and extracellular matrix.429 During dynamic contractions, especially those involving rapid lengthening or shortening, costameric proteins (e.g., integrins, dystroglycan complexes, vinculin) anchor titin and the Z-discs to the sarcolemma, minimizing the risk of sarcolemmal damage and promoting a more uniform distribution of mechanical stress.430 By linking the internal cytoskeleton to external support structures, costameres reduce localized strain and coordinate lateral force transmission, ultimately contributing to the muscle’s capacity for powerful yet resilient contractions.431
Residual force enhancement (RFE) is a phenomenon where the force generated by a muscle remains elevated after active lengthening (eccentric contraction) compared to isometric contractions at the same final muscle length.432 This property is thought to result from both active and passive components within the muscle.433 On a molecular level, RFE is influenced by the interaction between titin and actin and the stabilization of cross-bridges during and after lengthening contractions.434 During an eccentric contraction, the titin protein undergoes a conformational change that increases its stiffness and passive tension.435 This change is mediated by the binding of titin to actin in response to increased sarcomere stretch.436 Under high force conditions, segments of titin may interact more strongly with actin filaments, effectively increasing the stiffness of the sarcomere and enhancing passive force production.437 This increased stiffness allows the muscle to maintain higher levels of force without additional cross-bridge cycling, contributing to the residual force observed after the contraction.438
Furthermore, the cross-bridges themselves are thought to undergo a process of “catch bonding,” where the affinity of the myosin head for actin increases under tension, prolonging the attached state and enhancing force production during and after fast eccentric contractions.439 This behavior is attributed to structural changes in the myosin head induced by mechanical strain, which stabilize the actomyosin complex and resist detachment.440 The prolonged attachment of cross-bridges under tension allows for sustained force generation without a proportional increase in metabolic energy expenditure.441
The presence of RFE has significant implications for dynamic muscle function, as it allows muscles to maintain high levels of tension without expending additional metabolic energy.442 This property is particularly beneficial during activities involving repeated stretch-shortening cycles, such as running, jumping, or rapid deceleration, where efficient force production and energy conservation are critical.443 The ability to sustain elevated force levels after lengthening also plays a role in joint stabilization, helping to maintain muscle tension even when the muscle is being forcibly lengthened by external loads.444 This contributes to the prevention of excessive joint movement and protects ligamentous structures like the ACL from undue strain.445
Short-range stiffness refers to the muscle’s rapid (< 20ms)and pronounced resistance to small, sudden stretches (called preflexes) at the very start of force application.446 It hinges on the ability of cross-bridges to quickly engage and resist lengthening before significant muscle elongation occurs.447 This rapid resistance is facilitated by both neural and molecular mechanisms, including preactivation, phosphorylated regulatory proteins, and the readiness of myosin heads to attach to actin when calcium levels rise.448 Additionally, internal muscle structures such as titin, desmin filaments, and costameres provide a supportive framework that resists these minor stretches and distributes mechanical loads efficiently.449 This reflex-like stiffness is critical in high-velocity or high-impact activities, allowing muscles to counter unexpected perturbations and safeguard joints against sudden displacements.450 By stiffening almost instantly, muscles reduce the latency between neural command and physical response, aiding in rapid postural adjustments and finely tuned motor control.451
In broader functional terms, short-range stiffness is essential for energy efficiency and injury prevention.452 Because the muscle offers substantial resistance to small stretches through partially formed cross-bridges and elastic titin elements, it can store and return energy during activities involving rapid stretch-shortening cycles (e.g., running or jumping), reducing overall ATP expenditure.453 This energy-saving property complements the rapid force-generating capacity of skeletal muscle, helping athletes maintain performance over repeated bouts of high-intensity movement.454 Simultaneously, the muscle’s ability to resist small deformations early on helps stabilize joints and reduce excessive motion that could injure ligaments or surrounding tissues.455 These dual benefits—conserving metabolic resources and providing immediate mechanical protection—underline the significance of short-range stiffness as an integral element of efficient and safe muscle function.456
Activities such as sudden deceleration, cutting maneuvers, and awkward landings often load the knee joint so quickly that neural reflexes don’t have time to generate enough counterforce to prevent harmful knee displacements.457 Short-range stiffness, however, provides an immediate “buffer” against these abrupt stresses, reducing joint excursion during the critical 20–30 ms window before neural feedback can take over.458 By stiffening almost instantly, the muscles surrounding the knee—particularly the quadriceps and hamstrings—help limit anterior tibial translation and rotational instabilities that place the ACL at risk.459 Through this rapid mechanical response, muscles can absorb or redistribute energy that would otherwise be transmitted directly to the ligament.460 As a result, training programs designed to enhance muscle viscoelastic properties—such as plyometrics, overspeed eccentric exercises, and motor control exercises—can improve short-range stiffness and, in turn, bolster knee stability.461 This integrated response mechanism underscores the indispensable role that intrinsic muscle properties play in both everyday movement control and in preventing devastating ligamentous injuries like ACL tears.462
Understanding the molecular basis of intrinsic muscle properties is essential for optimizing neuromuscular control and improving joint stability during high-risk movements.463 Preactivation primes the muscle for rapid force generation, providing immediate resistance against destabilizing forces.464 Efficient cross-bridge kinetics ensure that muscles can produce force quickly and adapt to changing mechanical demands.465 Residual force enhancement contributes to sustained tension and stability after lengthening contractions, reducing the risk of uncontrolled joint motion.466 Alongside these processes, the role of desmin and costameres in lateral force transmission ensures that the internal contractile machinery remains mechanically integrated and well-supported under diverse loading conditions.467
Deficits in these molecular properties—such as delayed preactivation, inefficient cross-bridge kinetics, or reduced residual force enhancement—can lead to impaired neuromuscular control and increased susceptibility to injury.468 For example, a delayed preactivation response may result from impaired calcium handling, leading to insufficient muscle stiffness during initial foot-ground contact and allowing excessive anterior tibial translation, which places the ACL at risk.469 Similarly, slow cross-bridge kinetics may reduce the muscle’s ability to resist high-speed perturbations, compromising the rapid force production needed during sudden changes in movement.470 Diminished RFE can lead to inadequate stabilization during rapid deceleration or changes in direction, as the muscle may not maintain the necessary tension to control joint motion effectively.471 Moreover, disruptions in desmin filaments or costamere components can weaken the structural integrity of the muscle fiber, diminishing the muscle’s ability to transfer force and withstand mechanical stress.472
In conclusion, the molecular basis for intrinsic muscle properties is integral to understanding how muscles function during complex movements and how they contribute to joint stability.473 By targeting these properties through specific training interventions—such as plyometric exercises for enhancing preactivation, resistance training for optimizing cross-bridge kinetics, and eccentric exercises for increasing residual force enhancement—it is possible to improve muscle function at the molecular level.474 These interventions can lead to adaptations such as increased expression of fast-twitch myosin isoforms, enhanced calcium handling capacity, and modifications in titin stiffness, all of which contribute to more effective force production and joint stabilization.475 Strengthening or maintaining desmin filaments and costamere integrity further reinforces lateral force transmission, enhancing overall muscle resilience.476 Ultimately, a deeper understanding of the molecular biology underlying muscle function enables the development of strategies to reduce the risk of ligamentous injuries like ACL ruptures, improve overall athletic performance, and advance rehabilitation outcomes.477
10. Feedback and feedforward mechanism of ACL injury
The feedback and feedforward mechanisms are fundamental neurophysiological processes that underlie movement control and stability, playing a crucial role in protecting the knee joint from excessive loads and preventing anterior cruciate ligament (ACL) injury.478 These mechanisms operate synergistically to modulate muscle activation patterns, joint stiffness, and dynamic stability, ensuring that the musculoskeletal system can adapt efficiently to both anticipated and unexpected perturbations.479 When these control systems are impaired or insufficiently developed, the knee is more susceptible to entering positions of high mechanical stress, leading to an increased risk of ACL rupture. Understanding how these mechanisms interact and influence neuromuscular control is essential for both injury prevention and the development of effective rehabilitation strategies aimed at restoring optimal knee function (Figure 7).480
Feedforward control, often referred to as anticipatory control, involves the central nervous system’s (CNS) ability to prepare the body for an upcoming movement or external force before it occurs.481 This preparation is based on prior experiences, learned motor patterns, and predictions about the required muscular activity to achieve the desired movement outcome.482 Neurophysiologically, feedforward control is mediated by a complex network of brain regions, including the primary motor cortex, premotor cortex, supplementary motor area (SMA), basal ganglia, and cerebellum.483 These areas work in concert to generate and refine motor plans based on sensory inputs and internal models of body dynamics.
The primary motor cortex initiates voluntary movements by sending neural impulses through the corticospinal tract to lower motor neurons in the spinal cord.484 The premotor cortex and SMA are involved in planning and coordinating complex movements, integrating information about the position of the body in space and the timing of muscle activations.485 The basal ganglia contribute to the selection and initiation of appropriate motor programs, filtering out unwanted movements and ensuring smooth execution. The cerebellum plays a pivotal role in predicting the sensory consequences of movements and adjusting motor commands to minimize errors.486
A critical aspect of feedforward control is the generation of an efference copy, an internal copy of the motor command sent from the motor cortex to sensory regions of the brain, such as the cerebellum and parietal cortex.487 This efference copy allows the CNS to predict the expected sensory feedback from a movement and compare it with actual sensory input, facilitating rapid adjustments if discrepancies are detected.488 This mechanism enhances the efficiency of motor control by reducing reliance on slower feedback processes.
When a movement is planned, the CNS sends feedforward signals to the muscles, pre-activating them to optimize joint position and stiffness before the movement begins.489 This anticipatory activation is particularly important for stabilizing the knee joint during high-risk maneuvers such as cutting, jumping, or sudden deceleration, where the knee is exposed to rapid changes in direction and loading.490 Neurophysiologically, this involves increased excitability of alpha motor neurons innervating the stabilizing muscles, mediated by enhanced synaptic input from descending motor pathways.
During dynamic movements, the quadriceps and hamstring muscles are pre-activated in a coordinated manner to control knee joint positioning.491 The hamstrings, innervated by the sciatic nerve, play a vital role in counteracting the anterior shear forces generated by quadriceps contraction during knee extension, thereby protecting the ACL from excessive strain.492 The timing of muscle activation is critical; the hamstrings must be activated slightly before or concurrently with the quadriceps to effectively stabilize the tibia relative to the femur.
When feedforward control is impaired—due to factors such as fatigue, inadequate neuromuscular training, or previous injury—the timing and magnitude of muscle pre-activation are disrupted.493 Fatigue can alter neurotransmitter levels and receptor sensitivities at synapses within the CNS and peripheral nervous system, leading to decreased neural drive to the muscles. Additionally, changes in cortical excitability and synaptic efficacy can impair the transmission of motor commands.494 This disruption results in a less stable knee joint at the moment of ground contact, increasing the risk of valgus collapse or anterior tibial translation, which are common mechanisms of ACL injury.
Research using electromyography (EMG) has shown that individuals with ACL injuries or those at high risk often exhibit delayed or diminished feedforward activation of key stabilizing muscles.495 Neuroimaging studies have also demonstrated altered activation patterns in motor planning regions, suggesting that the CNS may be less effective at anticipating and preparing for high-risk movements.496 For example, reduced activation in the SMA and increased reliance on visual processing areas indicate a shift from automatic to more conscious movement control, which is slower and less efficient.
While feedforward mechanisms are proactive, feedback mechanisms are reactive, providing the body with real-time corrections based on sensory feedback from muscles, tendons, ligaments, and joint capsules.497 The feedback system operates through both spinal reflex pathways and supraspinal circuits that adjust muscle activity in response to unexpected changes in joint position, load, or velocity.498 This real-time modulation of muscle activity is crucial for maintaining joint stability, especially during rapid movements or when external forces threaten to destabilize the knee.
Sensory receptors embedded in the musculoskeletal system play a central role in feedback control.499 Muscle spindles, located within the muscle belly, detect changes in muscle length and the rate of stretch, sending afferent signals through type Ia and II sensory fibers to the spinal cord. Golgi tendon organs, situated at the muscle-tendon junction, sense changes in muscle tension and force production, transmitting signals via type Ib afferent fibers.500 Ligamentous mechanoreceptors, such as Ruffini endings and Pacinian corpuscles found in the ACL and other ligaments, respond to changes in joint tension and provide critical information about joint position and movement direction.501
When these sensory receptors detect a perturbation, they transmit afferent signals to the dorsal horn of the spinal cord.502 Here, they synapse onto interneurons and motor neurons, modulating motor output through reflex pathways. For example, the muscle spindle afferents facilitate the monosynaptic stretch reflex, leading to reflexive contraction of the stretched muscle.503 In the case of the knee, when sudden valgus stress or anterior tibial translation occurs, the sensory receptors within the ACL and surrounding tissues activate spinal reflexes that increase hamstring and gastrocnemius muscle activity to resist the destabilizing force.504 This spinal reflex loop, known as the ligamentous-muscular protective reflex, operates with minimal delay, providing an immediate response to sudden changes.
In addition to spinal-level reflexes, feedback control involves higher-order neural circuits that integrate sensory information at cortical and subcortical levels.505 The cerebellum is essential for refining feedback control by processing proprioceptive inputs and adjusting motor outputs accordingly. It receives sensory information via the spinocerebellar tracts and compares the actual movement with the intended movement as indicated by the efference copy.506 If discrepancies are detected, the cerebellum sends corrective signals to the motor cortex and brainstem nuclei to adjust muscle activation patterns, a process critical for maintaining balance and coordination.
The parietal cortex, particularly the posterior parietal cortex, integrates multisensory information to contribute to spatial awareness and body schema—the internal representation of body position in space.507 This region aids in the conscious perception of joint position and movement, informing voluntary motor adjustments. The basal ganglia also play a role in modulating feedback control by influencing motor planning and execution, particularly in the selection and initiation of appropriate motor responses based on sensory inputs.508
Following an ACL injury, there is often a disruption in the feedback control system, characterized by decreased sensitivity of mechanoreceptors due to damage or degeneration of sensory nerve endings within the ligament.509 This loss of proprioceptive input leads to altered central processing of sensory information, resulting in diminished accuracy of joint position sense and delayed reflexive responses.510 Functional MRI studies have shown changes in brain activation patterns after ACL injury, including reduced activity in sensory processing areas and increased reliance on visual and vestibular inputs. This shift may reflect compensatory mechanisms but can lead to increased reaction times and decreased agility.511
The interplay between feedforward and feedback mechanisms is essential for maintaining dynamic knee stability and preventing ACL injuries.512 In a healthy sensorimotor system, these two mechanisms work in harmony to optimize joint position, muscle stiffness, and force distribution. Feedforward control prepares the musculoskeletal system for expected demands, while feedback control allows for rapid adjustments to unforeseen changes.513 The cerebellum serves as a critical hub for integrating feedforward and feedback information, ensuring smooth and coordinated movements.
When one of these mechanisms is impaired, the other may attempt to compensate, often leading to suboptimal control strategies.514 For example, if feedforward pre-activation is deficient, the system may rely more heavily on feedback responses to maintain stability. However, reflexive responses are inherently slower than anticipatory activations and may not generate sufficient muscle force to counteract high-impact forces during rapid movements.515 Conversely, if feedback control is compromised due to sensory deficits or impaired reflex pathways, the CNS may increase feedforward activation in an attempt to stabilize the joint preemptively. This can result in increased muscle co-contraction and joint stiffness, which, while reducing joint laxity, may limit dynamic movement and elevate the risk of secondary injuries such as muscle strains.516
Effective prevention and rehabilitation programs should target both feedforward and feedback mechanisms to restore optimal neuromuscular control.517 Neuromuscular training that emphasizes anticipatory muscle activation, such as plyometric exercises, agility drills, and sport-specific movement patterns, can enhance feedforward control by improving the CNS’s ability to predict and prepare for high-risk movements.518 These activities promote neural adaptations, including increased synaptic efficiency and enhanced cortical and subcortical connectivity, leading to more effective motor planning and execution.
Proprioceptive and motor control training, including balance exercises, perturbation training, and joint position sense activities, can improve feedback control by enhancing the sensitivity of sensory receptors and refining reflexive responses.519 Such training stimulates the regeneration and plasticity of neural pathways associated with proprioception, facilitating better integration of sensory information and more accurate motor adjustments.520 Additionally, incorporating cognitive tasks during physical training can improve the ability to process multiple sources of information simultaneously, further enhancing neuromuscular control.
Overall, the feedback and feedforward mechanisms are integral to protecting the ACL and maintaining knee stability.521 Disruptions in these neurophysiological processes can compromise the body’s ability to respond effectively to mechanical stresses, increasing the susceptibility to injury. Addressing both proactive and reactive neuromuscular control in injury prevention and rehabilitation programs is crucial. By enhancing the CNS’s capacity for anticipatory activation and improving the responsiveness of sensory feedback systems, it is possible to reduce the risk of ACL injuries and promote optimal functional outcomes. Understanding the neurophysiological underpinnings of these mechanisms provides valuable insights for developing targeted interventions that restore and enhance the intricate balance of motor control required for safe and effective movement.
11. Long term negative adaptation post ACL injury
The long-term negative adaptations following an anterior cruciate ligament (ACL) injury extend far beyond the initial structural damage to the ligament and have profound implications on neuromuscular control, motor coordination, and overall functional performance. These adaptations are driven by a cascade of neurophysiological and biomechanical changes that can persist for months or even years after the injury, affecting the individual’s ability to perform complex movements and increasing the risk of re-injury or secondary complications.522
One of the most critical aspects of these long-term adaptations is the alteration in neurocognitive function, which manifests as slowed reaction times and decreased processing speed. After an ACL injury, the central nervous system (CNS) often requires more time to process sensory information and generate appropriate motor responses. This delay in cognitive processing is problematic during high-speed athletic movements, such as cutting or rapid changes in direction, where split-second decisions and fast motor execution are essential for maintaining joint stability.523
Another significant long-term adaptation involves the impairment of visuomotor integration. Visual-motor reaction time refers to the time it takes for the CNS to interpret visual stimuli and coordinate the appropriate motor response. Following an ACL injury, there can be disruptions in the integration of visual inputs with motor planning regions of the brain, resulting in slower response Times.524 This impairment can be particularly detrimental in sports where athletes rely heavily on visual cues to anticipate opponents’ movements or changes in the environment. A slower visual-motor reaction time may lead to delayed muscle activation and poor joint positioning during dynamic tasks, further exposing the knee to potentially harmful forces that could re-injure the ACL or damage other structures, such as the meniscus or collateral ligaments.525
At the neuromuscular level, long-term adaptations are evident in the altered electromechanical properties of the muscles surrounding the knee joint. There is often a persistent increase in electromechanical delay (EMD) in the quadriceps and hamstring muscles of individuals with a history of ACL injury.526 EMD refers to the time lag between the onset of electrical activity in the muscle and the actual generation of force. This delay indicates changes in the contractile and elastic properties of muscle fibers, as well as disruptions in neuromuscular transmission. Increased EMD reduces the muscle’s ability to generate rapid contractions, which are essential for stabilizing the knee during sudden perturbations. The prolonged EMD in the quadriceps and hamstrings suggests that these muscles are less effective at counteracting anterior tibial translation and rotational forces, placing greater stress on the passive structures of the knee, including the ACL graft or remaining ligamentous tissue.527
In conjunction with increased EMD, individuals with long-term ACL deficits often demonstrate a lower rate of torque development (RTD) in the quadriceps and hamstrings.528 RTD is a measure of how quickly a muscle can produce force and is critical for explosive movements such as jumping, sprinting, and rapid deceleration. A lower RTD means that the muscles are slower to produce the necessary forces to stabilize the joint, leading to a higher likelihood of joint instability and abnormal loading patterns. This reduction in RTD can be attributed to both neural and muscular factors, including decreased motor unit recruitment, changes in muscle fiber composition, and alterations in the stiffness of the muscle-tendon complex.529 As a result, athletes with a history of ACL injury may struggle to regain their pre-injury levels of performance and may compensate by altering their movement mechanics, such as relying more on the hip and ankle for stability, which can lead to additional injuries and chronic joint pain.
Another significant long-term adaptation is the deficit in postural control and balance. Postural correction refers to the body’s ability to make rapid adjustments to maintain balance and prevent falls when the center of mass shifts unexpectedly.530 After an ACL injury, there is often a decrease in proprioceptive feedback from the knee joint due to damage to the mechanoreceptors within the ligament. This loss of proprioception disrupts the sensory feedback loop that is essential for maintaining postural stability, leading to slower and less accurate postural corrections.531 Over time, this can result in compensatory strategies, such as increased reliance on visual and vestibular inputs to maintain balance, which are less effective during high-speed movements or when the athlete is fatigued. Impaired postural correction increases the risk of falls and non-contact injuries, as the body is unable to adapt quickly to changes in surface conditions or sudden shifts in body weight.
These chronic deficits in postural control are often accompanied by altered trunk and hip mechanics, which further destabilize the knee joint.532 The CNS may compensate for the impaired proprioceptive input from the knee by increasing activation in the hip abductors and core muscles, leading to abnormal kinematic patterns such as increased trunk sway, hip adduction, and pelvic drop. These compensations place additional stress on the knee joint and can lead to malalignment, such as dynamic valgus, which is a known risk factor for ACL re-injury.533 Over time, these altered movement patterns become ingrained, making it difficult for individuals to return to normal movement mechanics without targeted rehabilitation interventions.
Furthermore, chronic neuroplastic changes in the CNS contribute to these long-term adaptations. The decreased sensory input from the injured knee joint alters the representation of the limb in the primary somatosensory cortex, leading to a phenomenon known as cortical reorganization.534 This altered cortical representation is associated with deficits in proprioceptive acuity and motor control, making it difficult for individuals to accurately perceive joint position and initiate appropriate muscle activations.535 The loss of precise cortical representation also affects motor planning areas, such as the premotor cortex, leading to impaired motor sequencing and coordination during complex movements.
Long-term neurocognitive changes, such as reduced attentional capacity and increased reliance on conscious motor control, are also observed in individuals with a history of ACL injury.536 These changes reflect the brain’s adaptation to the altered sensory environment, where greater cognitive resources are required to perform movements that were previously automatic. This increased cognitive load can negatively impact performance, especially in high-pressure situations where quick decision-making and fast reactions are critical.537 The need for conscious control of knee movements also reduces movement efficiency and fluidity, making the athlete more prone to errors and poor movement strategies that increase injury risk.
In summary, the long-term negative adaptations following an ACL injury encompass a wide range of deficits, including slowed neurocognitive processing, impaired visuomotor integration, increased electromechanical delay, reduced rate of torque development, and impaired postural correction.538 These deficits are driven by a combination of neuromuscular, proprioceptive, and neurocognitive changes that alter the body’s ability to effectively stabilize the knee joint and respond to dynamic challenges.539 Addressing these long-term adaptations requires a comprehensive rehabilitation approach that targets not only strength and power but also sensory integration, motor coordination, and cognitive processing to restore optimal movement control and reduce the risk of re-injury.
12. Motor Learning Post ACL injury
Motor learning following an anterior cruciate ligament (ACL) injury is a complex, multi-phase process involving significant neurophysiological changes within the central nervous system (CNS). This process consists of three main phases: the initial phase, the learning phase, and the automatic phase.540 Each phase is characterized by distinct neural adaptations and alterations in cortical and spinal excitability. Understanding these phases is crucial for re-establishing neuromuscular control, coordinating dynamic knee stability, and optimizing movement patterns disrupted due to ligamentous damage and subsequent loss of sensory input—a phenomenon known as deafferentation (Figure 8).541
In the initial phase of motor learning post-ACL injury, the CNS undergoes significant reorganization to compensate for the sudden loss of proprioceptive feedback from the damaged ligament.542 The ACL contains mechanoreceptors—such as Ruffini endings, Pacinian corpuscles, and free nerve endings—that provide essential information about joint position and movement.543 When these receptors are damaged, the CNS receives diminished sensory input, leading to impaired joint position sense and kinesthesia.
This loss triggers neuroplastic changes in the brain’s motor and sensory cortices. Functional MRI studies have shown increased activation in areas like the primary motor cortex (M1), supplementary motor area (SMA), and premotor cortex.544 These regions become hyperactive as the brain attempts to recalibrate motor commands without reliable sensory feedback.545 The increased cortical activity reflects a shift from automatic to more conscious motor control, requiring greater cognitive effort to perform movements that were previously subconscious.
At the spinal level, there is altered excitability of motor neurons.546 The reduction in afferent input from the knee joint affects the monosynaptic reflex pathways, leading to decreased reflexive muscle activation. This impairment can result in delayed muscle responses and reduced muscle stiffness around the knee, compromising joint stability.547 Additionally, inhibitory interneurons may become more active due to pain and swelling, further suppressing motor neuron excitability and muscle force production.
During the learning phase, the CNS begins to form new motor patterns to adapt to the altered sensory environment.548 Neuroplasticity mechanisms, such as synaptogenesis and dendritic branching, are enhanced to create new neural pathways that can compensate for the lost proprioceptive input.549 The brain relies more heavily on visual and vestibular information to guide movements, integrating these senses to improve spatial awareness and coordination.
The cerebellum plays a crucial role in this phase by processing sensory inputs and fine-tuning motor outputs.550 It helps in error detection and correction, allowing the individual to adjust movements based on feedback. Motor learning involves strengthening synaptic connections through long-term potentiation (LTP), which increases the efficacy of neural transmission along frequently used pathways.551 Repetitive practice and rehabilitation exercises facilitate this process, reinforcing the neural circuits responsible for stabilizing the knee.
Corticospinal excitability increases as the motor cortex enhances its output to compensate for deficits in spinal reflexes. This heightened excitability leads to greater recruitment of motor units in the muscles surrounding the knee, such as the quadriceps and hamstrings.552 Enhanced muscle activation improves joint stiffness and dynamic stability, reducing the reliance on passive structures like the ACL graft.
In the automatic phase, the new motor patterns become more refined and require less conscious effort to execute. The CNS achieves a new level of efficiency in controlling knee movements, with decreased activation in higher-order cortical areas and increased reliance on subcortical structures like the basal ganglia.553 This shift reflects the transition from conscious, deliberate movements to automatic, well-coordinated actions.
Synaptic connectivity stabilizes, and the neural circuits involved in knee stabilization become more efficient. The strengthened synapses formed during the learning phase are maintained, ensuring consistent and reliable motor responses.554 At the spinal level, reflex pathways regain functionality, and muscle activation patterns normalize, contributing to improved proprioceptive acuity and joint position sense.
The cerebellum continues to play a role in coordinating movements but with less need for corrective adjustments. The reduced error signals indicate that the motor commands and sensory feedback are better aligned.555 As a result, the individual can perform complex movements with greater ease and confidence, minimizing the risk of re-injury.
Understanding these phases of motor learning is essential for designing effective rehabilitation programs post-ACL injury. During the initial phase, interventions should focus on restoring basic joint function and reducing inflammation and pain to minimize inhibitory effects on motor neurons.556 Gentle range-of-motion exercises and isometric contractions can help maintain muscle activation without overloading the healing tissues.
In the learning phase, rehabilitation should emphasize neuromuscular training that challenges the CNS to develop new motor strategies. Balance training, proprioceptive exercises, and controlled perturbations can enhance sensory integration and motor coordination.557 Task-specific exercises that mimic functional activities are crucial for reinforcing the neural pathways involved in daily movements and sports-specific tasks.
During the automatic phase, the goal is to consolidate motor skills and transition the individual back to normal activity levels. High-level functional training that includes agility drills, plyometrics, and sport-specific movements can help solidify the automaticity of new motor patterns.558 Ongoing assessment and refinement of technique are important to ensure that movement patterns are efficient and do not place undue stress on the knee joint.
Motor learning after an ACL injury involves a series of neurophysiological adaptations that progress through the initial, learning, and automatic phases. Each phase reflects the CNS’s remarkable ability to reorganize and adapt in response to altered sensory input and mechanical demands.559 By targeting these phases through tailored rehabilitation strategies, it is possible to restore neuromuscular control, improve dynamic knee stability, and optimize movement patterns.560 This comprehensive approach not only facilitates recovery but also reduces the risk of future injuries, enabling individuals to return to their desired activities with confidence and competence.
13. Visual-Cognitive Control to Chaos Continuum in Rehabilitation
The concept of the Visual-Cognitive Control to Chaos Continuum in rehabilitation is an advanced framework for understanding how motor control and sensorimotor integration evolve from structured, visually-guided movements to highly complex and chaotic scenarios that mimic real-world sport and dynamic environments.561 This continuum is based on the premise that the rehabilitation of injuries—especially those involving dynamic joints like the knee—requires a systematic progression that transitions from basic motor control, dependent on visual feedback and cognitive input, to more complex, unpredictable conditions where reflexive and automatic responses dominate. This approach is particularly relevant for athletes recovering from anterior cruciate ligament (ACL) injuries, as they must regain not only strength and stability but also the ability to perform at high speeds and under variable, chaotic conditions that characterize sports settings.562
In the early phases of rehabilitation, following an ACL injury or surgery, the focus is on re-establishing basic neuromuscular control and restoring joint stability through structured and controlled exercises.563 During this stage, patients often rely heavily on visual feedback and conscious cognitive control to execute even simple movements. This dependency arises because the proprioceptive input from the injured joint is diminished, making it difficult for the central nervous system (CNS) to accurately gauge joint position and movement. Visual feedback, therefore, becomes the primary source of information that guides motor actions, compensating for the loss of proprioceptive acuity. The heavy reliance on visual control is evident when patients watch their knee during exercises, consciously attempting to correct any deviations in alignment or balance.564
Cognitive involvement during this phase is also heightened, as patients must consciously think about how to execute movements that were previously automatic. This cognitive control is necessary to ensure proper technique and to prevent the adoption of compensatory strategies that could lead to maladaptive movement patterns.565 The high cognitive demand, however, limits the speed and fluidity of movement, as the brain must allocate additional resources to control the knee’s position and stability. Exercises at this stage are usually performed in a stable, controlled environment, with minimal external perturbations or variability, allowing patients to focus on accuracy and technique. Tasks such as single-leg balance exercises, controlled squats, and step-ups are often used to re-establish a foundation of neuromuscular control.566
As rehabilitation progresses and proprioceptive function begins to improve, the emphasis shifts toward integrating visual and proprioceptive inputs to enhance the CNS’s ability to coordinate movements without over-reliance on visual cues.567 During this phase, patients are encouraged to perform exercises that challenge their balance and stability without the constant use of visual feedback, such as closing their eyes during balance tasks or focusing on an external target. The goal is to enhance the brain’s ability to use proprioceptive information from the muscles, tendons, and ligaments to maintain joint stability.568
In this stage, more complex movements, such as single-leg hops, agility drills, and reactive stepping, are introduced. These exercises require the integration of visual and proprioceptive feedback to respond to changes in body position and external stimuli, such as a moving target or a changing Surface.569 The cognitive demand remains relatively high, as the patient must process and integrate multiple sources of sensory information to control their movements. However, as proficiency increases, the CNS begins to rely less on visual input and more on internal proprioceptive cues, allowing for faster, more fluid movements. This phase represents the transition from a purely visual-cognitive control strategy to one that is more balanced between visual, proprioceptive, and vestibular systems.570
The advanced phase of rehabilitation moves the patient along the continuum towards exercises that incorporate increasing levels of complexity, unpredictability, and environmental variability.571 At this point, the focus is on enhancing the athlete’s ability to perform complex movements in response to unexpected stimuli or chaotic environments that more closely resemble game-like conditions. Introducing chaos into the rehabilitation setting—such as through randomized perturbations, sudden changes in direction, or reactive agility drills—forces the CNS to rely on feedforward control and reflexive responses, rather than purely visual or cognitive strategies.572 This adaptation is critical because, in real-world scenarios, athletes rarely have the opportunity to plan their movements with perfect visual guidance; instead, they must respond quickly and accurately to unpredictable events, such as an opponent’s movements or an uneven playing Surface.573
The integration of reactive drills that simulate sport-specific scenarios, such as sudden cutting maneuvers, defensive pivots, or unplanned jumps, challenges the CNS to generate appropriate motor responses without the benefit of advanced planning.574 For instance, an agility drill that involves responding to random visual or auditory cues—such as changing direction in response to a coach’s signal or evading a moving obstacle—places a high demand on the visual, vestibular, and proprioceptive systems to work in concert. This chaotic environment mimics the real-time decision-making and rapid motor adjustments that athletes need on the field, helping to restore not only strength and stability but also the cognitive-motor flexibility necessary for safe return to sport.575
A critical component of this continuum is the role of cognitive-motor coupling, which refers to the interplay between cognitive processes—such as attention, decision-making, and sensory processing—and motor execution. During rehabilitation, as the athlete progresses from structured, visually-guided exercises to more chaotic, dynamic movements, the demands on cognitive resources shift.576 In the early phases, a high level of cognitive engagement is required to consciously control movements, but as automaticity increases, the focus shifts toward developing cognitive resilience, where the athlete can maintain effective motor control even under conditions of cognitive stress, such as fatigue or multitasking.577
This aspect of training is particularly important because many ACL injuries occur not only due to mechanical factors but also due to cognitive overload or distraction.578 Introducing dual-task exercises, such as performing agility drills while simultaneously counting backward or responding to a secondary visual stimulus, trains the brain to process multiple sources of information without compromising motor performance. This dual-task training enhances the athlete’s ability to maintain motor control under pressure, reducing the likelihood of a sensorimotor breakdown that could lead to re-injury.579
The final stage of the Visual-Cognitive Control to Chaos Continuum involves refining reflexive control to the point where the athlete can perform complex, unpredictable movements with minimal conscious effort.580 At this stage, exercises are designed to push the limits of the athlete’s reactive capacity, challenging the CNS to generate rapid, precise motor responses in highly variable and unpredictable conditions. This phase is characterized by the integration of external distractions, unanticipated perturbations, and sport-specific drills that mimic the chaotic nature of competitive environments.581 The athlete is exposed to a variety of unpredictable scenarios, such as sudden opponent movements or unexpected changes in terrain, that force the CNS to rely on reflexive, feedforward, and automatic responses.582
The objective of this phase is to ensure that the athlete can maintain dynamic stability and proper joint alignment even when cognitive resources are limited or when unexpected events occur.583 Reflexive control is achieved through repeated exposure to chaotic environments, which trains the CNS to quickly adjust motor commands based on minimal sensory input.584 By the end of this phase, the athlete should be able to perform at a high level of intensity, speed, and complexity without a conscious focus on the injured knee, reflecting a full return to sport-specific automaticity and resilience.585
In summary, the Visual-Cognitive Control to Chaos Continuum provides a structured framework for progressing from visual and cognitive reliance to reflexive, automatic control in chaotic environments.586 This continuum is essential for restoring not only the physical capacity of the injured knee but also the neuromuscular and cognitive abilities required for safe, high-performance athletic activities.587 Successful navigation through this continuum ensures that the athlete’s sensorimotor system is fully prepared for the demands of real-world sports, minimizing the risk of re-injury and optimizing long-term outcomes.
14. Eccentric Training: Rehabilitation Following an ACLR.
Resistance training is an integral component of athletes’ physical preparation.588 It is well documented that resistance training can improve a range of neuromuscular variables—muscle strength, power, and endurance—that are essential for high athletic performance and top performance results.589,590 Traditional resistance training typically includes both eccentric and concentric phases. Eccentric muscle actions involve the active lengthening of muscle tissue against an external force or load,591 in contrast to isometric and concentric muscle actions, which don’t involve a change in muscle length or involve a shortening of muscle tissue. Use of eccentric training in physical preparation leads to numerous neuromuscular adaptations and uniquely affects muscle architecture, which can indirectly or directly influence athletic performance592 (Figure 9).
Eccentric training can be effectively used in the rehabilitation process after ACLR (Anterior Cruciate Ligament Reconstruction).593 ACL injuries are among the most common in sports, affecting both men and women.594,595 Eccentric muscle action predominantly occurs during deceleration and changes of direction.596 Deceleration training in team sports can potentially reduce the incidence of ACL injuries.597 A significant number of non-contact injuries occur during deceleration and changes of direction. In soccer matches, rapid horizontal deceleration while defending or pressing is among the main movement patterns that lead to lower-limb injuries, including ACL ruptures.598
A comparison of athletes with high and low eccentric quadriceps strength showed that those with higher eccentric strength had a significantly greater ability to generate horizontal braking forces.599 The two-joint rectus femoris muscle is the key quadriceps muscle responsible for absorbing eccentric forces during deceleration, especially when the trunk is more upright. When the trunk leans forward, due to large horizontal deceleration forces, the gluteus maximus and hamstrings likely contribute to controlling the external hip-flexion moment as well as attenuating and distributing forces. During matches, abrupt horizontal decelerations in defensive play are among the main situational patterns commonly associated with severe lower-limb injuries, such as ACL tears.600
According to Suchomel T., four main methods of eccentric training can be distinguished601:
- Tempo Training
- Flywheel Training
- Accentuated Eccentric Loading
- Plyometric Training
Each of these eccentric methods can effectively contribute to ACLR rehabilitation and prevent ACL injuries. Research suggests that early implementation of tempo training can positively influence the hypertrophy and strength of the muscles surrounding the knee after ACL reconstruction.602 Flywheel training can be applied both in the rehabilitation process following ACLR and in preventing ACL injuries.603 Flywheel training is a relatively new method used in continuous-resistance exercise with eccentric overload. Exercises performed in this manner lead to improvements in strength and power, hypertrophy, muscle activation, muscle length, and tendon stiffness. Other positive effects of flywheel training include significant athletic enhancements in areas such as speed, jump height, and change of direction.604 These positive outcomes can be attributed to the eccentric and power-specific characteristics of the training, making flywheel training ideal for musculoskeletal rehabilitation.605 Flywheel training can be used to prevent injuries, reintroduce training after periods of unloading, rehabilitate tendons and muscles, as part of postoperative rehabilitation, during late-stage sports rehabilitation, and also to prevent falls and treat sarcopenia in older adults.606
Although there are many ways to incorporate these methods into ACLR rehabilitation at various training phases, there is currently a lack of evidence-based recommendations regarding the optimal way to implement each method.
15. Implicit Learning in ACL Rehabilitation: Bridging Rehab and Sports Performance
In competitive sports, athletes execute complex movements while making split-second decisions in unpredictable environments—far removed from the controlled settings of traditional rehabilitation. Standard rehab often relies on task-oriented exercises and intrinsic feedback and explicit learning, which may reinforce maladaptive motor patterns and increase re-injury risk.
To better prepare athletes for sport-specific demands, implicit learning and dual-task approaches are essential, even in early rehab. The goal of exercise repetition is not to enable players to perform nearly identical movements, but to consistently realize the goal of movement in changing environmental conditions. Rather than repeating identical movements, athletes must develop adaptable solutions to achieve movement goals under varying conditions—“repetition without repetition.” This approach improves adaptability and reduces reliance on rigid, idealized techniques.
16. Implicit Learning and Dual-Task Integration
Implicit motor learning strategies aim to minimize explicit knowledge of movement execution. Instead of focusing on how a movement is performed, implicit learning methods intent to direct attention toward the goal of the movement. This approach reduces working memory demands during motor coordination, freeing cognitive resources for performance-related decision-making.607 In high-pressure, complex sports environments, implicit learning has been shown to:
-
Reduce cognitive load, enhancing performance under physical and mental stress.608
-
Improve retention and transfer of motor skills to real-world scenarios.609
Combining neuromuscular control exercises with neurocognitive tasks promotes implicit learning by shifting focus toward the intended effect of a movement (goal-directed attention), rather than internal bodily awareness (self-directed attention). This dual-task integration engages both perceptual-cognitive and physical performance factors, mirroring the functional demands of sport.610
17. Examples of Implicit Dual-Task Exercises
Exercise 1: 30 lights total, each color is coupled with a different movement task. Colors green (+1), red (-1), yellow (+2) and purple (-2) also require counting. The patient adds the values and reports the total score at the end of the set.
-
Red: Step-up (Right Leg): Pass left; Count -1
-
Green: Single-leg Squat (Right Leg): Pass left; Count +1
-
Purple: Single-Leg RDL (Right Leg): Count -2
-
Blue: Step-up (Left Leg): Header
-
Yellow: Single-leg Squat (Left Leg): Header; Count +2
-
White: Single-Leg RDL (Left Leg)
This is a complex motor-cognitive dual task. Beginners can start with a simplified version using four colors and no counting. Gradually, the task can be progressed by increasing the number of colors and adding the counting challenge to enhance difficulty.
Exercise 2: Perform low-intensity plyometric jumps while tracking moving balls on the Skillcourt screen. Depending on the difficulty level, 1 or 2 balls must be tracked among several moving on the screen. For 5–8 seconds, the athlete performs rapid lateral jumps until the balls stop moving. Once they stop, the athlete moves to the square that matches the final position of the tracked ball(s).
Exercise 3: This dual-task rehab exercise integrates the Beat Saber VR Game as a visuo-cognitive challenge for ACLR rehabilitation. The patient wears a VR headset and maintains balance in a single-leg stance while playing the game. Using VR lightsabers, the patient cuts through glowing blocks, with difficulty levels and game settings tailored to suit different skill levels and rehab phases.
-
Early Rehab: The patient balances on one leg while slicing blocks, combining proprioceptive training with a cognitive task.
-
Advanced Rehab: The game incorporates faster rhythms and lateral movement to deflect incoming objects. These tasks can be further combined with jumping or lateral agility, challenging the body to react dynamically.
The adaptability of the game makes it an excellent visuo-cognitive motor task throughout various ACLR rehab phases, progressing from balance-focused to high-intensity agility training.
These tasks combine motor control, perception, and decision-making, creating sport-relevant training environments.
18. The Brain-Body Connection in ACL Injuries
ACL injuries disrupt neural processes involving visual, proprioceptive, and attentional integration.611 Athletes with high-risk landing mechanics post-ACL rehab often exhibit altered brain activation patterns, characterized by increased reliance on visual-proprioceptive processing and heightened attentional demand for movement coordination.
While these patterns may temporarily compensate for injury, they can compromise neuromuscular control in high-pressure scenarios involving opponents or ball-related actions—common ACL injury mechanisms.612 Alarmingly, task-oriented rehab may inadvertently reinforce these maladaptive brain activation patterns rather than correcting them.
To address this, rehab programs must simulate the unpredictable, decision-rich environments athletes face during competition. By integrating perception, decision-making, and neuromuscular control into training, clinicians can retrain both the brain and body, reducing injury risk and improving sport readiness.613
Conclusion
ACL injuries are not simply a mechanical failure of the knee joint but represent a complex disruption of the sensorimotor system, involving profound changes in neural connectivity, sensory processing, and motor control. Traditional rehabilitation methods that focus solely on strengthening the musculature around the knee often overlook the critical role of the CNS in coordinating dynamic joint stability. Neuroplastic therapy, employing tools like external focus strategies, stroboscopic glasses, smartboards, and virtual reality, offers a comprehensive solution that addresses both the neuromuscular and neurocognitive deficits associated with ACL injuries. By engaging the CNS in targeted exercises that progressively challenge visual, proprioceptive, and reflexive controls, this approach enhances neural plasticity, improves motor learning, and accelerates the restoration of dynamic stability. Through systematic integration of these tools across different phases of rehabilitation, neuroplastic therapy not only aids in the re-establishment of stable, automatic movement patterns but also prepares athletes for the chaotic and unpredictable nature of sports environments. This advanced approach ensures that athletes return to their sport at a higher level of proficiency, with greater confidence and a reduced risk of re-injury, ultimately optimizing long-term outcomes and performance.