Introduction
Caudal epidural steroid injections (ESIs) are a widely accepted interventional procedure for the management of chronic lumbosacral pain, particularly in patients with radiculopathy secondary to lumbar disc herniation, spinal stenosis, or post-laminectomy syndrome.1 Administered via the sacral hiatus, caudal ESIs offer a route to deliver corticosteroids and local anesthetics to the epidural space to reduce inflammation, improve function, and decrease pain-related disability.2–4
Compared to interlaminar and transforaminal approaches, caudal ESIs are often considered technically less complex. They may carry a lower risk of complications, particularly in patients with altered spinal anatomy or those who have undergone prior spinal surgeries.5,6 However, as with all epidural procedures, there remains concern for some potential adverse events, including dural puncture, post-dural puncture headache, infection, bleeding, and the rare but serious risk of permanent neurological injury.7–10
Despite their frequent use in clinical practice, there remains limited recent data precisely characterizing the safety profile of caudal ESIs in routine outpatient settings. The purpose of this retrospective review is to evaluate the incidence of documented complications, including dural puncture and permanent neurological injury, in a random sample of patients who underwent caudal ESIs over six months.
This study aims to contribute to the growing body of literature supporting the safety and utility of this commonly performed procedure.
Methods
A retrospective chart review was conducted to evaluate the safety profile and clinical outcomes of caudal ESIs performed at a single outpatient pain management clinic. The review included 40 randomly selected cases performed over six months from July 1, 2024, to December 31, 2024.
Inclusion criteria consisted of adult patients (age ≥18 years) who received a caudal ESI for treating lumbosacral pain or radiculopathy. Patients who received alternative epidural approaches (e.g., interlaminar or transforaminal) were excluded. Only patients with complete follow-up data—either from two-week clinic follow-up visits or post-procedure phone calls—were included in the outcome analysis. Patients with incomplete follow-up were excluded from pain score and outcome assessments.
Board-certified pain medicine physicians performed all procedures under sterile technique and fluoroscopic guidance. The injectate used in all cases included a corticosteroid (commonly triamcinolone acetonide or dexamethasone), a local anesthetic (typically 1–2% lidocaine or 0.25% bupivacaine), and preservative-free normal saline. Proper epidural spread was confirmed via fluoroscopy in all cases.
Electronic medical records were reviewed for documentation of procedural complications, including dural puncture, post-dural puncture headache, infection, neurological deficits, or other adverse events. Pain scores before and after the injection and patient-reported percent relief were extracted when available. Data collection and analysis were descriptive in nature, with the primary outcome being the incidence of documented dural puncture or permanent neurological injury following caudal ESI.
Results
A total of 40 patient charts were reviewed over a six-month period, each corresponding to a caudal ESI performed for lumbosacral pain or radiculopathy. There were no documented cases of dural puncture, post-dural puncture headache, infection, or permanent neurological injury among the reviewed cases.
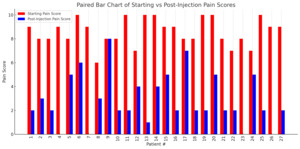
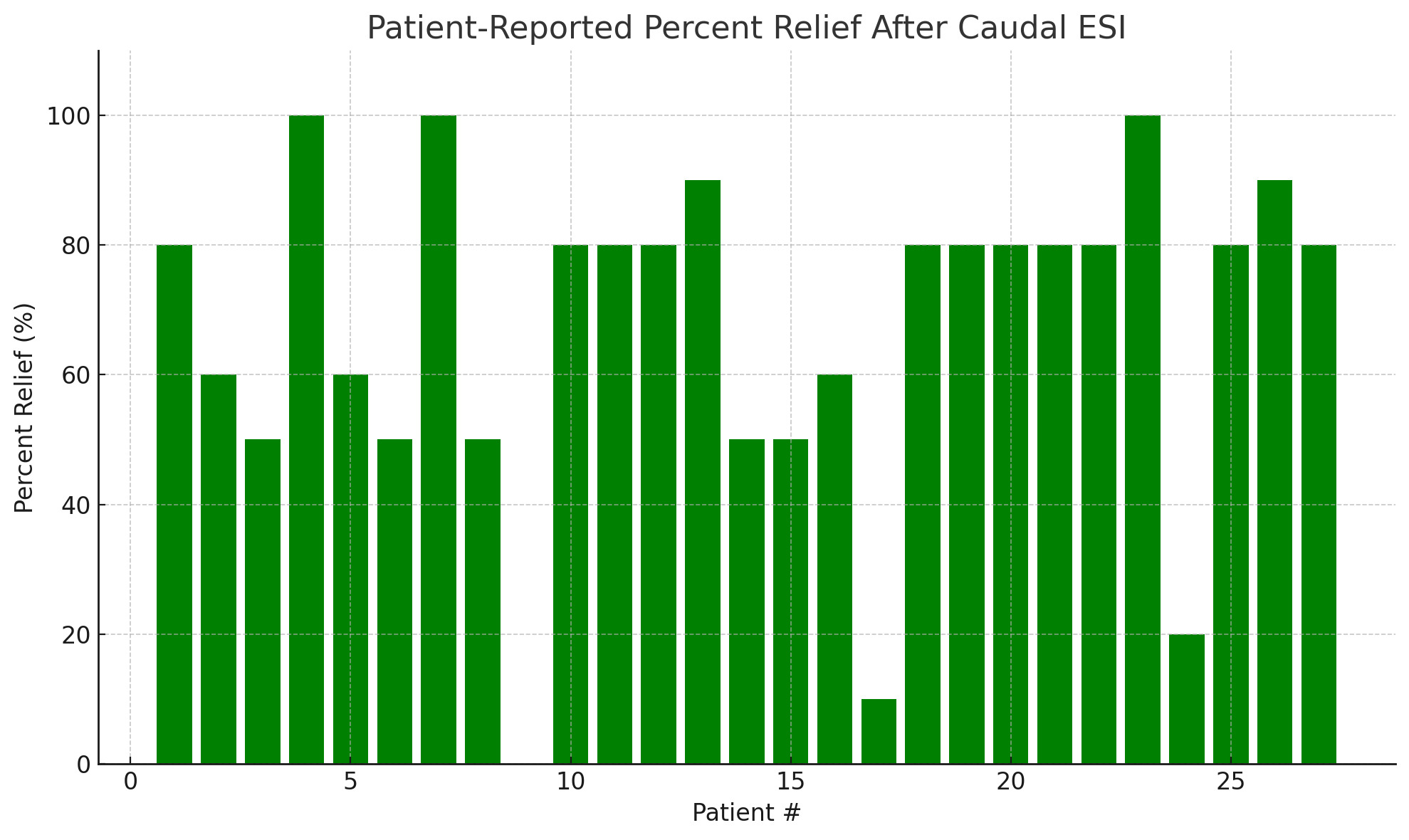
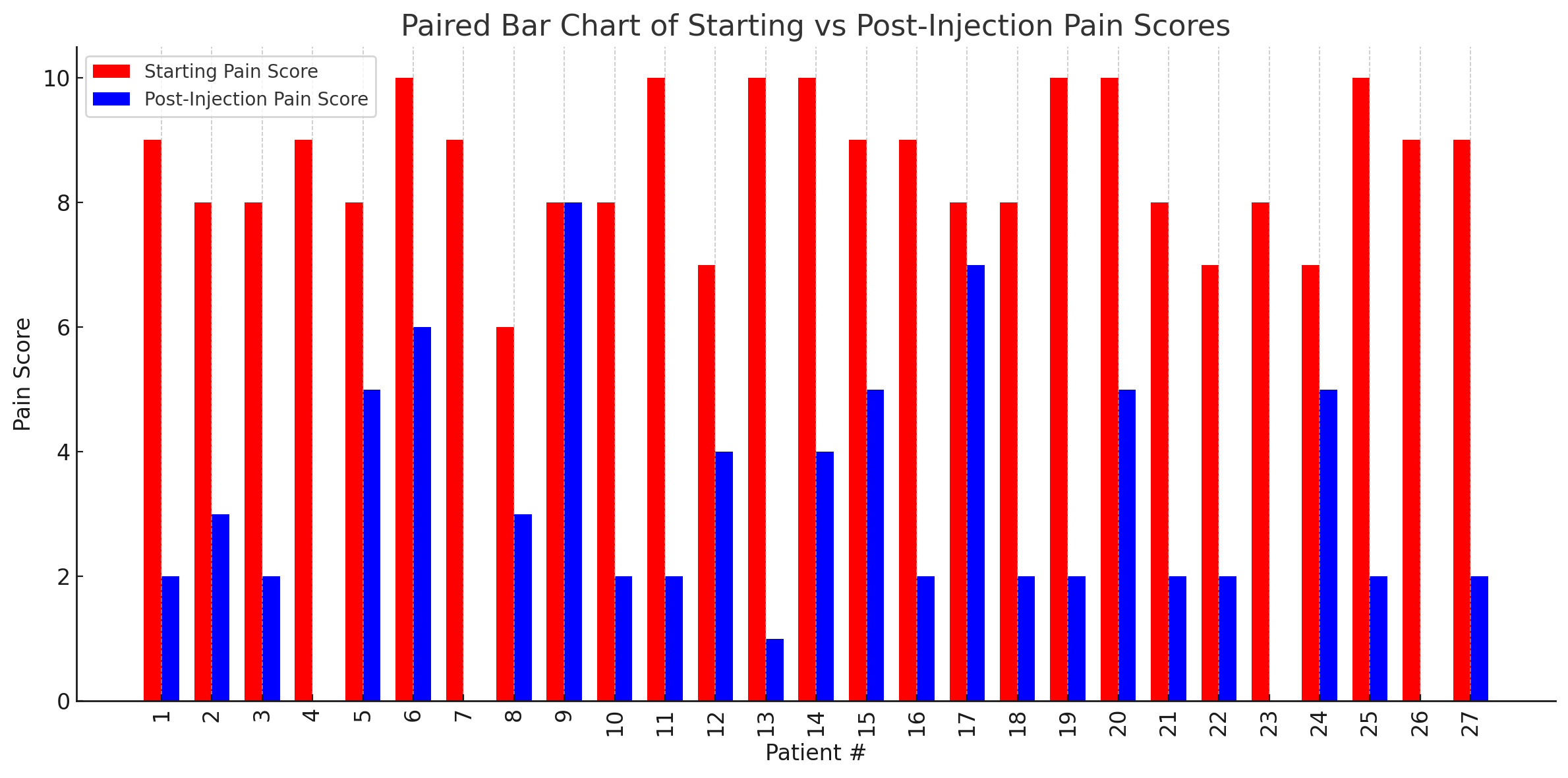
Of the 40 charts, 27 contained complete data regarding pre- and post-procedure pain scores on a numerical rating scale (NRS) as well as patient-reported percent pain relief. The average starting pain score across these patients was 8.6 (range: 6–10), which decreased to an average post-injection pain score of 2.9 (range: 0–8). The average patient-reported percent relief was 67.4%.
A total of 24 patients (88.9%) reported at least 50% relief in symptoms following the procedure. Three patients (11.1%) reported complete (100%) relief, while only one patient reported no relief (0%). A summary of individual patient pain scores and relief percentages is presented in Table 1.
A visual representation of the individual patient-reported percent relief following caudal ESI is provided in Figure 1.
Figure 2 presents the patient-reported NRS pain scores before and after caudal ESI. This side-by-side comparison highlights the reduction in pain scores experienced by most patients following the procedure.
These findings support the safety and potential effectiveness of caudal ESIs in providing meaningful short-term symptom relief in patients with lumbosacral pain.
Discussion
This retrospective review of randomly selected caudal ESIs performed over six months demonstrated a favorable safety profile, with no documented cases of dural puncture or permanent neurological injury. These findings suggest that the caudal approach is among the safest routes for epidural steroid administration, particularly in patients with altered lumbar anatomy or a history of prior spinal surgery.5,11,12
Among the 27 patients with complete outcome data, caudal ESI was associated with meaningful short-term pain relief. The average reported pain score decreased from 8.6 to 2.9, and the average patient-reported percent relief was 67.4%. Notably, 88.9% of patients reported at least 50% improvement in symptoms, and 11.1% (3 patients) reported complete (100%) pain relief. Only one patient reported no relief following the procedure. These findings highlight the potential clinical benefit of caudal ESI for appropriately selected patients suffering from lumbosacral radicular pain or axial low back pain.
The absence of complications in this series highlights the relative safety and technical ease of the caudal approach when performed in patients with typical anatomy. This technique is generally straightforward. However, anatomical variations such as abnormal sacral curvature or obesity can make needle placement more challenging, particularly when image guidance is not used. Fluoroscopy, especially when paired with contrast, is critical in confirming proper needle placement and ensuring appropriate epidural spread of injectate. Additionally, reviewing pre-procedure imaging can assist in procedural planning and may help identify anatomic challenges that could affect outcomes.13
The lack of significant complications in this case series likely reflects the consistent use of fluoroscopic guidance, adherence to sterile technique, and standardized injection protocols. These findings are consistent with the existing literature. For example, a survey conducted by Brown et al. reported that among 86 interventional pain physicians, none reported significant neurological injuries related to caudal ESIs, reinforcing the safety of this commonly performed procedure.5
Together, these results reinforce the safety and short-term effectiveness of caudal epidural steroid injections when performed under image guidance by experienced practitioners. The consistency of these findings with previously published literature further supports the role of caudal ESI as a reliable and low-risk interventional option for managing lumbosacral pain. While the outcomes observed in this series are encouraging, they must be interpreted within the context of the study’s design and scope.
Limitations
This study has several limitations that should be considered when interpreting the findings. The sample size was primarily relatively small, with only 40 cases randomly reviewed and 27 patients included in the outcome analysis due to incomplete follow-up data. This limits the generalizability of the findings. However, this limited sample size is expected for a pilot study and serves as a preliminary step toward larger, more comprehensive investigations. Additionally, the follow-up data were collected at varying intervals through either clinic visits or post-procedure phone calls, which may introduce inconsistency in how and when patient outcomes were assessed. Outcomes were limited to short-term pain relief without assessment of long-term effectiveness, functional improvement, or quality-of-life measures.
Despite these limitations, our findings reinforce the safety and short-term efficacy of caudal ESIs in the outpatient setting. Future studies with standardized follow-up intervals, larger patient cohorts, and long-term outcome tracking are warranted to validate these results further and guide clinical practice.
Conclusion
Caudal ESIs appear to be a safe and effective interventional option for the management of lumbosacral pain and radiculopathy in the outpatient setting. This retrospective review of 40 cases identified no significant complications such as dural puncture or permanent neurological injury. Among the patients with complete follow-up data, the majority experienced meaningful pain relief, with an average reduction in pain scores and a high percentage of patients reporting at least 50% symptom improvement.
These findings support using caudal ESIs as part of a multimodal pain management strategy. Further prospective studies with larger patient populations and long-term follow-up are needed to confirm these results and better define the duration and predictors of therapeutic benefit.