Introduction
Tears of the hip abductor muscles (gluteus medius and minimus) commonly cause lateral hip pain, hip abductor weakness, and gait instability, especially in middle-aged to elderly women. Despite abductor tendon tears being quite common, with estimates that they are present in as many as 20% of patients with hip arthritis, this pathology is often misdiagnosed as trochanteric bursitis or Greater Trochanteric Pain Syndrome.1 Despite its significant impact on patients’ daily lives, conservative measures such as physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroid injections are often recommended. When these interventions fail, and imaging reveals the presence of an abductor tendon tear, surgical repair is the next line of intervention.2 Extensive evidence suggests that both open and endoscopic repair significantly decrease pain levels.3,4 For example, a prospective study on 34 patients with endoscopic gluteus medius tear repair showed significant improvements in both the Harris Hip Score (HHS) and Non-Arthritic Hip Score (NAHS), as well as a notable reduction in pain scores over 2 years postoperatively.5
Despite the encouraging results, suboptimal healing is often observed in patients with significantly retracted or chronically generated tears, particularly those with fatty infiltration of the abductor muscles, and in particular the gluteus medius.6 To address this issue, researchers have begun exploring the use of biological augmentation to facilitate tendon healing. With advancements in biological augmentation for rotator cuff repairs, these same materials have been proposed for use on gluteus medius tendons to enhance their structural integrity and improve clinical outcomes.7 However, there hasn’t been any published data on the volume of cases for which biological augmentations are used in gluteus medius tendon tears.
Biological augmentation includes the use of autologous blood derivatives such as platelet-rich plasma (PRP) and platelet-rich fibrin matrix (PRFM), cellular and structural support materials, including bone marrow aspirate concentrate (BMAC), collagen scaffolds, acellular dermal matrices, and synthetic implants.8 These biological materials facilitate tendon healing and regeneration by nurturing growth factors and progenitor cells, while scaffolding materials are designed to provide structural support to the repair. There has been an increase in the use of these biological augmentation tools in gluteus medius tear repairs in recent years9; the use of bioinductive collagen patches in particular, initially developed for rotator cuff repairs, has become more frequently used in hip abductor tendon repairs.
This systematic review aimed to quantify the published clinical evidence supporting the use of these different biological augmentation techniques in the context of gluteus medius tear repair, specifically PRP, PRFM, collagen patches, dermal allografts, synthetic scaffolds, and BMAC, on postoperative healing, function, and complication rates.
Methods
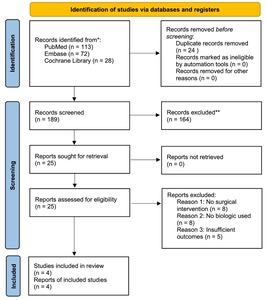
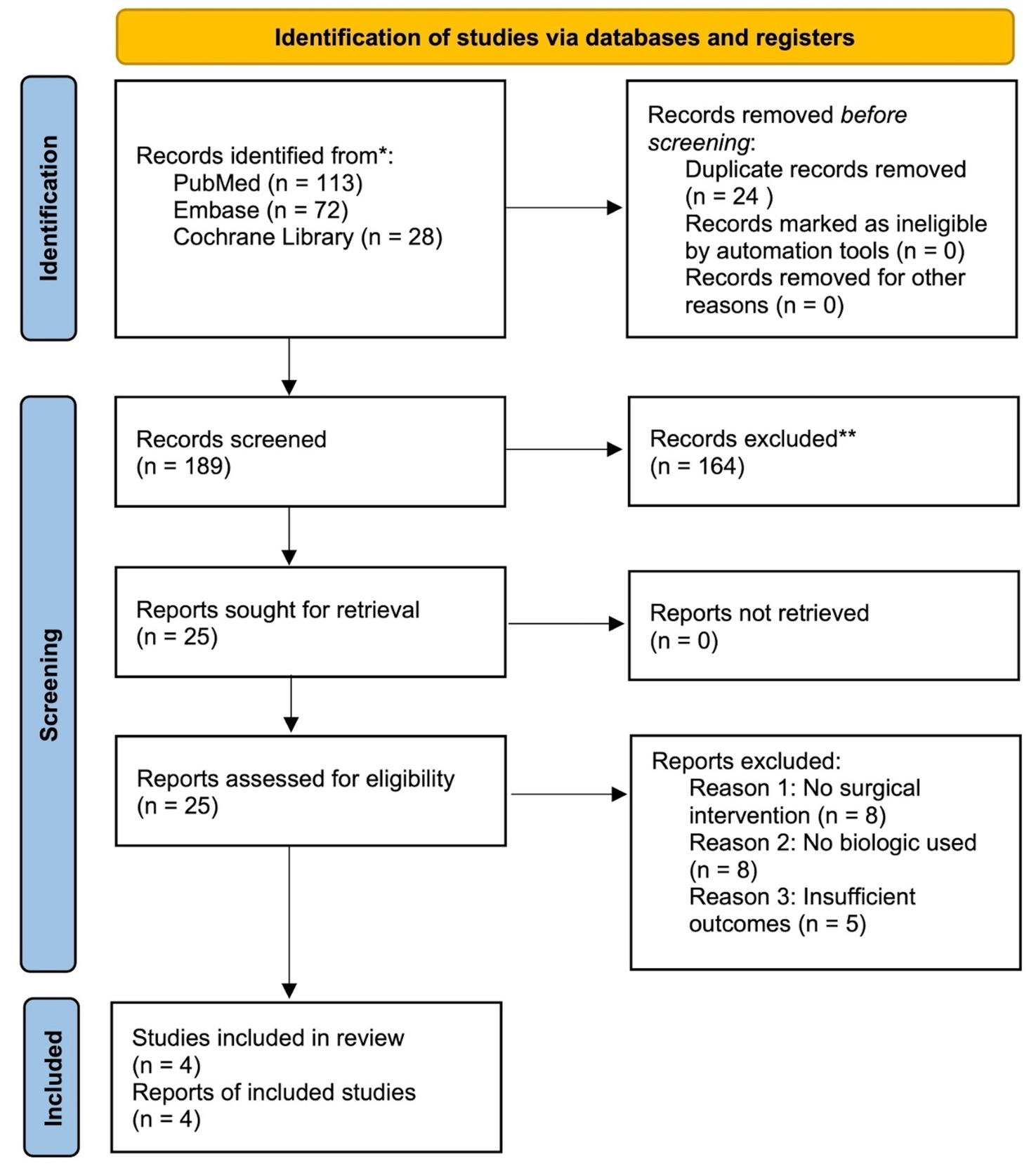
We used PubMed, Embase, and Cochrane Library databases to identify publications reporting on the clinical outcomes of bio-augmentation for gluteus medius tear repairs, spanning the period from January 2000 to March 2025 inclusive. The keywords used for the search included: “gluteus medius”, “hip abductors”, “tendon repair”, “platelet-rich plasma”, “platelet-rich fibrin”, “collagen patch”, “acellular dermal matrix”, “scaffold”, “bone marrow aspirate concentrate”, and “stem cells”. Inclusion criteria included the following: (1) the inclusion of human subjects with gluteus medius tear repair; (2) at least one type of biologic augmentation used intraoperatively; (3), reporting of clinical outcomes such as pain scores, functional assessments, imaging studies, and the rate of re-tears. Study designs include randomized controlled trials, prospective and retrospective cohort studies, case-control studies, and case series. In the initial screening, all studies that did not contain an in vivo surgical component were excluded, as well as basic science experiments, animal studies, or editorials (Table 1). Due to the limited number of eligible clinical studies, two additional papers that did not meet full inclusion criteria were included in the narrative discussion to provide supplementary context. The formal evidence synthesis and quality grading did not include these studies.
All identified studies were first filtered using the title and abstract, followed by a full-text review of studies that passed the initial filtering (YL, RK, MA). For every included study, several key parameters were extracted, including patient demographics, tear characteristics (size and chronicity), surgical approach (open or endoscopic), the type of biological augmentation applied, follow-up time, and reported clinical outcomes. These outcomes include pain level, hip specific functional scores, radiographic evidence of tendon healing, retear rate, and the need for re-operations. We also included a table with a summary for the 4 included studies (Table 2). Based on the Oxford Center for Evidence-Based Medicine guidelines, each of the four study was assigned a level ranging from Level I (high-quality randomized controlled trials) to Level IV (case series and poor-quality cohort or case control studies). These assigned confidence levels were included in Table 2. In addition, the two supplementary studies that did not meet full inclusion criteria are summarized separately in Table 3 to provide additional narrative context. Due to the variety of study designs, interventions, and outcome measures, no meta-analysis was performed. Instead, findings were presented qualitatively.
Results
Our literature search yielded a surprisingly limited number of clinical studies that evaluated the impact of biological augmentation techniques on gluteus medius tendon repairs. No randomized controlled trials directly comparing augmentation strategies were identified. Available data primarily came from small retrospective cohort studies and case series, and insufficient data was available to conduct a meta-analysis. Key findings by augmentation types are summarized below.
I. Platelet-rich plasma (PRP)/Platelet-rich fibrin matrix (PRFM)
No studies directly examined PRP injection use in gluteus medius tendon tear repairs. However, there have been studies on PRP injection in treating abductor tendinopathy. Fitzpatrick et al10 conducted a level I RCT in which ultrasound-guided injection of leukocyte-rich PRP significantly improved the mean modified Harris Hip Score (mHHS) at 12, and 24 weeks post-injection (PRP: 74.05; control: 67.13; p=0.048) in patients with chronic gluteal tendinopathy (identified using a combination of characteristic symptoms, physical examination findings, and supported by ultrasound to confirm tendon pathology and excluded full-thickness tears) compared to corticosteroid injections. However, it is important to note that the study population did not include full-thickness tears or surgical patients.
One study evaluated intraoperative PRFM augmentation. Saltzman et al11 performed a retrospective comparative study of 47 patients undergoing endoscopic gluteus medius repair in which 18 patients received PRFM augmentation and 29 did not. Baseline characteristics and intraoperative variables were similar. At ≥ 12 months follow-up, both groups showed improvement in pain and hip function. However, multivariate analysis revealed no statistically significant differences in postoperative pain (VAS) (P=0.1), Hip Outcome Scores (ADL: P=0.31 or Sports: P=0.33), or mHHS (P=0.18). There was also no significant difference in clinical retear rates (P>0.5). Regression analysis did find slightly higher postoperative 12-Item Short Form Survey (SF-12: effect size, 0.651, P=0.016) and Physical and International Hip Outcome Tool-12 (iHOT-12: effect size, 0.467, P=0.029) scores in the PRFM group, suggesting a modest subjective physical benefit. Overall, Saltzman et al11 concluded that PRFM does not appear to influence gluteus medius repair outcomes with respect to pain or retear, although there may be modest improvement in some functional quality-of-life scores. In summary, PRFM augmentation was safe but did not confer a clear advantage in this series. No adverse events were reported in the study.
II. Bioinductive collagen patch
A bovine-derived collagen scaffold (“bioinductive patch”) has been used to augment tendon repair by providing a template for tissue ingrowth. Day et al12 reported a prospective series of 9 patients with symptomatic gluteus medius tears who underwent open, double-row repair augmented with a collagen patch. Pre-operatively, all patients had both clinical and MRI evidence of partial- or full-thickness tears (four high-grade ≥50% tears and five low-grade <50% tears). Preoperative MRIs and scores (mHHS, Hip Outcome Score: Activities of Daily Living (HOS-ADL), Hip Outcome Score Sport (HOS-Sport), iHOT-33) were recorded. At 6 months postoperatively, 7 of 9 tendons were completely healed by MRI criteria. The mean tendon dimensions increased significantly with the mediolateral thickness grew by 5.8 mm (P<0.001) and the anteroposterior dimension by 4.1 mm (P=0.02), yielding a cross-sectional area increase of 48.4 mm² (P=0.001). The gluteal muscle cross-sectional area did not change significantly (P>0.05). All outcome scores improved from baseline to 6 months, with mean gains in HOS-ADL, mHHS, and iHOT-33 exceeding published MCID values (all with P<0.05). No complications or retears were reported. The authors concluded that open repair with collagen patch augmentation was safe and associated with increased tendon thickness and promising early functional improvement. As this study lacked a control group, no direct comparison to a non-augmented technique can be made.12
III. Acellular dermal allograft
Acellular dermal matrix grafts have been used to reinforce abductor tendon repairs. Nadeau et al13 reported a retrospective cohort of 64 patients undergoing endoscopic gluteus medius repair by a single surgeon; 26 had standard suture repair (GMR), and 38 had repair augmented with acellular dermal allograft (GMR-A). The surgeon reserved allograft for cases with poor intraoperative tendon quality. Demographics and tear characteristics were similar. Follow-up was longer in the non-augmented group (39 vs. 24 months). At a minimum 12 months follow up, both groups had significant postoperative improvements from baseline, and there were no statistically significant differences in any patient-reported outcomes: Visual Analogue Scale (VAS) pain (3.3 vs. 3.3), University of California, Los Angeles (UCLA) activity, mHHS, HOS Sport Specific Subscale (HOS-SSS), or Single Assessment Numeric Evaluation (SANE) (all P>0.5). A higher proportion of the standard-repair group achieved the Patient Acceptable Symptom State (PASS) on the UCLA score (64% vs 34%; P=0.02), though PASS and minimally clinically important difference (MCID) achievement rates were similar. One patient in each group (3.8% GMR, 2.6% GMR-A) required revision repair with allograft at final follow-up. No graft-related complications (e.g. rejection or infection) were reported. The authors concluded that clinical outcomes were comparable with and without dermal allograft augmentation.13 However, as patient selection was biased towards patients with smaller tears not being treated with augmentation, it is unclear if in routine cases the use of the augment confers any advantage. In addition to this controlled series, various surgical technique reports have described dermal graft use in massive tears. For example, some surgeons apply an acellular dermal patch over a repaired tendon gap or use it in a “parachute” fashion to span defects. Browning et al14 described a patient with a superior gluteal reconstruction for an irreparable tear using dermal allograft and tendon transfer, noting good early results.
IV. Synthetic scaffolds
Synthetic materials (e.g. woven polymers) have been used experimentally to augment massive abductor repairs, but evidence is sparse. Jimenez-Telleria et al15 described a novel reconstruction technique in three patients with chronic, retracted abductor ruptures. Their technique transferred a portion of the gluteus maximus as a “V” flap and reinforced it with a synthetic polyethylene terephthalate mesh scaffold before anchoring it to the femur. At a mean 18-month follow up, all three patients showed marked improvement: mHHS rose from 31.8 to 75.6, mean abduction strength improved to 3/5 on average, and there was no residual Trendelenburg gait. The VAS score fell from 8.3 to 1.6. No complications were reported. While encouraging, this series lacks a control group and is too small to draw broad conclusions. There was another study mentioned about the usage of mesh augmentation in a revision surgery context, particularly when the gluteus medius was in continuity with the vastus lateralis.16 To our knowledge, no other studies have evaluated other synthetic scaffolds (e.g. polyester or PEEK patches) commonly used in the shoulder in gluteal repair. Thus, synthetic augmentation remains experimental and of unproven benefit, though it shows promise in complex reconstruction with large defects.
V. Bone marrow aspirate concentrate (BMAC)
BMAC has been injected for trochanteric tendinopathy with reported promise, but their role in augmenting tendon repair has not been studied in humans. There was a study published in Brazil that reported that BMAC injections yielded greater improvement in chronic gluteal tendinopathy than steroids, but this did not involve surgical repair.17 Therefore, BMAC remains an untested augmentation strategy in the repair of abductor tears.
Discussion
This systematic review highlights that high quality evidence for biologic augmentation in gluteus medius repair is extremely limited and underlines the need for more prospective studies. No randomized trials were found, and only a few small retrospective comparative studies exist. Overall, the current data does not convincingly support the routine use of PRFM or dermal allograft augmentation in routine tears. Saltzman et al11 found that PRFM, which is essentially a fibrin-based platelet scaffold, did not improve most objective outcomes. Similarly, Nadeau et al13 found no difference between repair with or without a dermal graft.
These findings temper expectations compared to promising results in rotator cuff repairs, though the latter has been more thoroughly studied. In the shoulder literature, PRFM has had mixed results18 and dermal patches often improve structural outcomes with less clear patient benefit.19 The absence of demonstrable benefit in gluteal studies may be attributable to patient selection bias; biologic augmentations were frequently employed in cases with poor tendon quality and poor expected outcomes. The finding that these patients did as well as patients with smaller tears, suggests significant benefit might accrue to patients with larger tears, but further study is needed to confirm this finding.
By contrast, the collagen patch study by Day et al12 suggests bioinductive implants could improve healing. Allograft-based patches are thought to stimulate host tissue remodeling and vascular ingrowth. In their 9-patient series, improved tendon thickness and high MRI healing rates were observed. However, without a control group, it is unclear if similar healing might have occurred with standard repair. Furthermore, this study’s follow up period was only 6 months. Additional studies with larger cohorts and longer follow-up are needed before concluding benefit.
The case series of synthetic mesh augmentation demonstrates that massive chronic tears can be reconstructed with reasonable results. The “flap-plus-mesh” technique showed large gains in strength and pain relief,15 likely due to the mechanical support of the mesh. However, synthetic scaffolds carry potential risks such as foreign body reaction and infection, and their long-term durability is unknown. Mesh augmentation may be indicated in situations where repair is otherwise not feasible.
Several factors complicate interpretation of the literature in a meta-analysis. First, study heterogeneity is high. Surgical techniques varied (open vs endoscopic, single-row vs double-row), as did rehabilitation protocols and concurrent procedures (many patients underwent hip arthroscopy for intra-articular pathology). Outcomes were assessed with different scales and at different times. Most studies included small sample sizes (typically fewer than 40 patients per group) and limited follow up durations with less than 2 years which substantially reduced statistical power. All comparative studies were retrospective in design, rendering them susceptible to selection and reporting biases. Furthermore, none of the studies blinded patients or outcome assessors to augmentation status, further limiting the internal validity of their findings. Only a couple of studies directly compared with a control group. In addition, publication bias may also be present, favoring reports of positive outcomes.
Biologically, augmentations aim to address the challenge of tendon-to-bone healing or the biomechanical performance of the tendon repair. PRFM provides a fibrin scaffold loaded with concentrated growth factors, but its effect may be modest if the underlying biology of the tendon is not responsive. In Saltzman et al11 study, PRFM did not reduce pain or retears, showing mixed results seen in rotator cuff augmentation. Dermal allograft adds collagen matrix to reduce gap formation and subsequent repair failure; however, if the native tendon repair is already strong and healing capacity is adequate, the graft may not be necessary.13 Collagen patches such as the bovine tendon scaffolds are designed to induce new tendon-like tissue regeneration at the repair site. The positive imaging findings in Day et al12 series support this mechanism of action. Nevertheless, the cost of such implants is high, and their routine use demands clear outcome benefits.
Notably, none of the augmentation studies reported significant safety issues: there were no infections, immune reactions, or re-operations directly attributable to the grafts or biologics. This suggests that augmentations are generally safe to be applied for gluteus medius tendon repairs. Thus, the decision to augment may rest on potential efficacy versus cost and complexity. As of now, high quality evidence to guide this decision is lacking.
The findings of this review must be considered in light of its limitations. We identified only a few relevant studies, all of which were low evidence level. The heterogeneity of the chosen studies precluded meta-analysis. Language restriction to English may have missed some data internationally. Additionally, some relevant abstracts or unpublished data such as conference proceedings might exist but were not captured. Our conclusions are therefore necessarily tentative but underline the necessity for more research in this space, particularly as awareness of the prevalence and underdiagnosis of this disease entity increases in orthopedics.
Conclusions
While biologic augmentation techniques have become more common in gluteus medius tendon repair, the current body of evidence offers limited support for their effectiveness. Comparative studies have not demonstrated meaningful improvements in clinical outcomes over routine repairs. There is weak indication that mechanical support may be helpful in larger, chronic tear repairs. Given these limitations, no single biologic augmentation approach can be routinely recommended. Future prospective trials are needed to determine whether these interventions can significantly enhance tendon healing or functional outcomes in gluteal abductor repairs.
Author’s contributions
Y.L. was responsible for the conception of the study, literature review, and drafting of the manuscript. M.A. and R.K. were responsible for assisting with the literature search, and manuscript revisions. S.B. provided senior supervision and contributed to critical revisions of the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
Dr. Stefano A. Bini received royalties from Stryker, and consulting support from Zimmer Biomet. He has stock or stock options in InSilicoTrials.com, CaptureProof.com, Cloudmedix.com, GateScience Inc., and SiraMedical.com; received research support from Google.com; received financial or material support from Elsevier; is on the editorial or governing board of The Journal of Bone and Joint Surgery, The Journal of Arthroplasty, and Arthroplasty Today; and is a board or committee member of the Personalized Arthroplasty Society and the Personalized Arthroplasty Foundation.
Y.L., M.A., and R.K have no conflicts of interest.
Funding
The authors did not receive any funds, grants, or other forms of support.
Acknowledgements
The authors have no acknowledgements to report.