INTRODUCTION
Rotator cuff injuries are among the most prevalent causes of shoulder pain and disability, particularly affecting two distinct populations: older adults with age-related degenerative tears and younger individuals—especially athletes—with traumatic or overuse-related injuries. In adults over 50 years of age, the prevalence of rotator cuff tears exceeds 25%, underscoring the significant burden on aging populations.1,2 These injuries often lead to pain, limited range of motion, and impaired function, severely impacting quality of life and daily activities.3
Although surgical repair remains an option—particularly for full-thickness tears or when conservative treatment fails—non-surgical management is frequently preferred due to its lower risk profile, cost-effectiveness, and accessibility.4,5 Among the non-operative modalities, injectable therapies have become increasingly utilized. Corticosteroid injections (CSIs), a long-established treatment, are primarily used for short-term pain relief due to their potent anti-inflammatory effects. However, repeated use has raised concerns regarding potential adverse effects on tendon integrity, including weakening and an increased risk of rupture.6,7
In contrast, platelet-rich plasma (PRP) injections have emerged as a biologic alternative aimed at enhancing tissue healing rather than solely reducing inflammation. PRP is an autologous blood product processed via centrifugation to yield a high concentration of platelets rich in bioactive molecules, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β).8,9 However, it is important to recognize that PRP is not a uniform therapy; it encompasses a range of formulations that vary in leukocyte content, platelet concentration, and activation methods—all of which may influence clinical outcomes.10 Moreover, treatment protocols also differ in terms of the number and timing of injections (i.e., single versus multiple sessions), further complicating interpretation and cross-study comparisons.
Despite the growing adoption of PRP in musculoskeletal medicine, its comparative efficacy relative to corticosteroids in managing rotator cuff tendinopathy remains controversial. While some trials and reviews suggest that PRP offers superior long-term improvements in pain and function, others report no significant differences between the two treatment modalities.11,12 These inconsistencies may stem from heterogeneity in study design, PRP preparation techniques, outcome measures, and patient populations.
Given these uncertainties, a systematic review and meta-analysis are warranted to synthesize the current evidence. This study aims to compare the efficacy and safety of PRP versus corticosteroid injections in patients with rotator cuff tendinopathy or partial-thickness tears. Special emphasis will be placed on outcome measures such as pain reduction, functional improvement, and adverse event profiles, while also accounting for variations in PRP formulation and injection protocols. By addressing these factors, this review seeks to provide a comprehensive, evidence-based perspective to guide clinical decision-making.
METHODOLOGY
Review of the literature
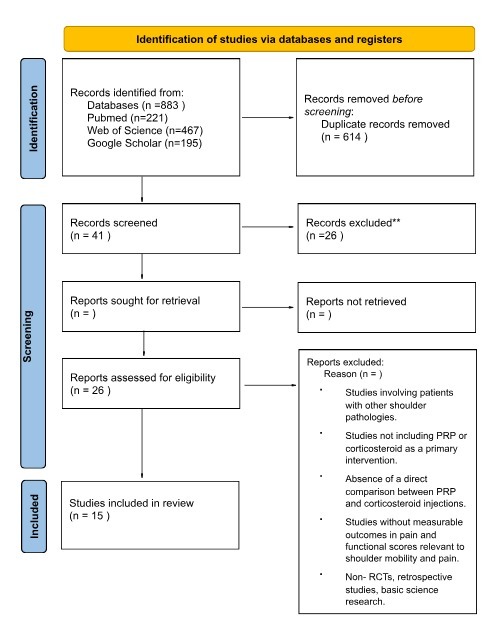
A comprehensive search of the following databases (PubMed, Web of Science Google Scholar, Cochrane, MEDLINE, EMBASE, and CENTRAL) up to December 2024 was done using the following search strategy: (Rotator cuff OR shoulder injury OR tendinopathy OR shoulder pain) AND (platelet-rich plasma OR PRP OR autologous conditioned plasma) AND (corticosteroid OR steroid OR cortisone). A total of 883 articles were included, and after duplicate removal 614 studies were subjected for title and abstract screening. Our review adhered to the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analyses) guidelines to minimize the bias in the selected studies.12 The study protocol was prospectively documented and registered in PROSPERO under the following ID: CRD42024616633.13 As this is a systematic review and meta-analysis study an ethical approval was not required.
Methodology for selecting studies
In our review, we included all studies that meet the following criteria: (1) studies published in English language, (2) studies that included adults 18 years old and above who were diagnosed with rotator cuff disease for at least three months, (3) Randomized Control Trials (RCTs) that compared the clinical outcomes of Platelet-Rich Plasma (PRP) injections and corticosteroids injection for rotator cuff disease (4) studies that reported measurable clinical outcomes including pain (VAS score), functional scores (e.g Constant-Murley, ASES, SST), and (5) it had to be randomized control trials.
Exclusion criteria include (1) studies that included shoulder pathologies outside rotator cuff disease, (2) studies that did not include PRP or corticosteroid injections as a primary intervention, (3) studies that did not report direct comparison between PRP and corticosteroid injections, (4) studies without measurable clinical outcomes in pain and functional scores relevant to shoulder mobility and pain, (5) non-RCTs such as reviews, editorials, or case reports and (6) studies that were not published in English.
Process for selecting and data extraction
Three Independent Reviewers (Nwaf Alshahir, Emtinan Fallatah, Mohammed Alsherieqi) have Screened Studies independently and simultaneously by title and abstract through Rayyan search web and mobile app for Systematic Reviews,14 after that three authors (Mishari Alanazi, Abdulaziz Mahdi, Nwaf Alshahir) have independently and simultaneously reviewed them by Full text, after which two authors have extracted data from papers (Talal Alassaf, Samar Alrajhi) for the following Variables, (1) Total Number Of Patients. (2) Number of Patients in PRP group, (3) Number of patients in Corticosteroid group, (4) Mean Age of Patients (5) Mean age of Patients in PPR group, (6) Mean Age in Corticosteroid group, (7) Number of males in PRP group, (8) Number of males in Corticosteroid group, (9) Number of females in PRP group, (10) Number of females in Corticosteroid group, (11) Mean Follow up in PRP group, (12)
Mean follow up in Corticosteroid group, (13) VAS score in PRP group Pre Injection,(14) VAS score in PRP group Post Injection (15) VAS score in Corticosteroid group Pre injection,(16) VAS score in Corticosteroid group Post injection (17) ASES in PRP group Pre Injection, (18) ASES in PRP group Post Injection,(19) ASES in Corticosteroid group Pre Injection, (20) ASES in Corticosteroid group Post Injection,(21) CMS in PRP group Pre Injection, (22) CMS in PRP group Post Injection, CMS in Corticosteroid group Pre Injection, (23) CMS in Corticosteroid group Post Injection.
Function Scores and MCID interpretation
The functional scores were evaluated using the Constant Murley Score (CMS), the American Shoulder and Elbow Surgeons Shoulder Score (ASES. Each score was examined as a deviation from the initial measurement at short-term (3–6 weeks), intermediate-term (8–12 weeks), and long-term (more than 12 weeks) follow-up assessments.15
Pain
Pain perception was assessed using the Visual Analog Scale (VAS) questionnaire score, which was analyzed as a deviation from the initial measurement at short-term (3–6 weeks), intermediate-term (8–12 weeks), and long-term (more than 12 weeks) follow-up assessments.15
Bias Assessment,
The Cochrane risk tool for bias was used to evaluate the quality of RCTs.16 This tool has various domains, and the judgments within each domain were carried forward for an overall RoB2 judgment across five main domains. These domains are fixed, focusing on aspects of trial design, conduct and reporting using a series of ‘signalling questions’ to elicit information relevant to the risk of bias. This is then judged using an algorithm, and the judgments can be ‘low’ (for all domains, the risk of bias is low), can express ‘some concerns’ (for at least one of the domains, there is some concern) or ‘high’ (for at least one domain has a high risk or some concerns for multiple domains). The risk of bias assessment was conducted independently by two authors (Mohammed Altorki, Hawra Abbas), and disagreements were resolved with consensus after consultation with senior author (Nwaf Alshahir).
Results
Comparison of PRP Injection and Steroid Injection on VAS Outcomes
A meta-analysis was conducted across seven studies to compare the efficacy of Platelet-Rich Plasma (PRP) injections and steroid injections on Visual Analog Scale (VAS) outcomes at 3-6 weeks. The overall mean difference in VAS scores between PRP and steroid injections was 1.22 (95 % Confidence interval (CI): 0.30 : 2.15) in favorable results toward PRP.
However, there was a significant heterogeneity (τ² = 0.60, chi-square = 16.07, df = 6, P = 0.01) (Figure 1A). After 12 weeks, the mean difference in VAS scores between PRP and steroid injections among 7 studies was 0.12 (-0.61 to 0.86), with moderate heterogeneity (τ² = 0.49, df = 6, P = 0.004, I² = 68%), and the overall effect was not significant (Z = 0.33, P = 0.74), indicating minimal difference in VAS outcomes between the two interventions at this time point (Figure 1B). After 24 weeks, the mean difference in VAS scores among 6 studies was 0.10 (-9.68 to 9.88), with very high heterogeneity (τ² = 141.78, df = 5, P < 0.000001, I² = 100%), and the overall effect was not significant (Z = 0.02, P = 0.98), suggesting no substantial difference between PRP and steroid injections after 24 weeks following the injection (Figure 1C).
Comparison of ASES Outcomes Between PRP and Steroid Injections
In addition to VAS, the American Shoulder and Elbow Surgeons (ASES) score was measured as another key outcome across the same time intervals. After 3-6 weeks, the mean difference in ASES scores between PRP and steroid injections was -1.94 (-10.69 to 6.82), with significant heterogeneity (τ² = 84.49, df = 4, P = 0.00001, I² = 87%), and the overall effect was not significant (Z = 0.43, P = 0.66), indicating no clear difference in ASES outcomes between the two treatments at this early stage (Figure 2A). However, after 12 weeks, the mean difference in ASES scores was 13.24 (1.26 to 25.23), with substantial heterogeneity (τ² = 171.90, df = 4, P < 0.000001, I² = 94%) and the overall effect was significant (Z = 2.17, P = 0.03), suggesting that PRP injections lead to a better improvement in ASES scores compared to steroid injections after 12 weeks following the injections (Figure 2B). Similarly, after 24 weeks, the mean difference was 11.23 (0.06 to 22.40), with significant heterogeneity (τ² = 112.19, df = 3, P < 0.000001, I² = 100%). The overall effect was marginally significant (Z = 1.97, P = 0.05), indicating that PRP injections might result in better long-term outcomes in ASES scores compared to steroid injections (Figure 2C).
Comparison of Constant-Murley Outcomes Between PRP and Steroid Injections
The Constant-Murley score was also evaluated at different time points. The mean difference in Constant-Murley scores at 3-6 weeks was -3.97 (-10.36 to 2.42) and after 12 weeks it was 5.60 (-2.23 to 13.43) with no significant significance (Z = 1.22, P = 0.22 and Z = 1.40, P = 0.16), suggesting no clear advantage of either treatment in improving Constant-Murley outcomes at this time point (Figure 3A and B). However, after 24 weeks, the mean difference in Constant-Murley scores was 9.85 (3.76 to 15.94), with high heterogeneity (τ² = 49.75, df = 5, P < 0.000001, I² = 91%), and the overall effect was significant (Z = 3.17, P = 0.002), indicating that PRP injections show KKU a significant improvement in Constant-Murley outcomes compared to steroid injections at the 24-week follow up (Figure 3C).
DISCUSSION
This systematic review and meta-analysis aimed to evaluate and compare the outcomes of PRP Injection and Steroid Injection in the management of rotator cuff injuries. The results of our study do not align with the previous literature comparing PRP and steroid injections in Rotator cuff injuries improvement.
In our Review, a total of 15 randomized controlled trials were included. The main findings of the study were that at 3-6 weeks PRP showed better Pain (VAS) Outcomes demonstrated by better short-term improvement in pain compared to steroids, however there was no significant difference at 12 and 24 weeks. PRP also showed no difference in the functional (ASES) scores at the 3-6 weeks period but showed a marginal improvement at the 12 weeks and 24 weeks period indicating better intermediate to long-term outcomes compared to steroids. When it comes to the shoulder-specific function (Constant-Murley) score, PRP also demonstrated better long-term outcomes compared to steroids as it showed significant improvements at 24 weeks, but no difference was noted at 3-6 and 12 weeks. Pre- and post-injection analyses indicated that both treatments were effective in reducing pain and improving function, but PRP showed more consistency over time. While steroids may provide short-term relief, PRP appears to be better for long-term pain management and functional recovery.
As addressed in previous studies, PRP showed superiority compared to steroids injection in long-term functional improvement and tendon healing. In this study, however, the findings have shown an efficacy of PRP in short-term pain management or VAS, but long-term effect in a duration of 12 weeks, and 24 weeks was not significant.
In addition to VAS, overall functional improvement has shown better outcomes in PRP compared to steroid injections. As recorded, the ASES score is also not significant in short-term duration. However, according to the result of our study, there is a significant improvement in ASES score in the durations of 12 and 24 weeks compared to steroid injections. In addition, the Constant-Murley score even though did not show significance in either groups in short term duration (6 weeks), it showed a better outcomes in favorable to
PRP at 24 weeks duration
Although specific subgroup analysis for athletes were limited, studies that involved athletic populations reported improved outcomes of PRP due to higher physical demand and healing potentials. Some RCTs included in the review appear to have more active patients in the PRP group other than the steroid group.17 Though, further research is needed to draw robust conclusions about PRP efficacy in athletic populations.
In addition, age variation in Anvesh et al.18 where younger patients are involved in the PRP group, potentially skewing the results in favor of PRP. affecting the quality of the results. Older adults may exhibit diminished responses to PRP due to age-related decline in tissue regeneration capacity.
Limitations
Several limitations should be addressed in this review. Even though the search strategy was extensive and thorough, non-English publications were not included. Another limitation of this study is that about half of the studies included were assessed as having a high risk of bias. This limitation was unavoidable due to the nature of the intervention. Moreover, the intervention protocol varied from one study to another, which included the PRP preparation, type of PRP, dose, technique, US guidance, anesthesia, etc. This Could be explained by the high heterogeneity observed in the meta-analysis. Variations in interventions are to be anticipated, given that PRP is preserved as a relatively new therapeutic approach. This resulted in inconsistencies in treatment regimen across various studies. It is recommended that future research focus on identifying the optimal PRP formulation and protocol for the treatment of shoulder tendinopathy. Furthermore, future studies should implement a more precise classification of patients, as the effectiveness of injection therapies may differ among patients with partial, massive, or incomplete rotator cuff tears, as well as those with tendinopathy or subacromial bursitis.
Conclusion
This systematic review and meta-analysis compared the efficacy of PRP injections and corticosteroid injections in the management of rotator cuff injuries based on pain and functional outcomes. The findings suggest that PRP provides better short-term pain relief 3-6 weeks compared to corticosteroids, as evidenced by improved VAS scores. However, no significant differences were observed between the two treatments at longer follow-up periods 12 and 24 weeks. Functional improvement, assessed using the ASES score, showed no significant short-term differences, but PRP demonstrated superior outcomes at 12 and 24 weeks. The CMS also favored PRP at 24 weeks, indicating better long-term shoulder function. While corticosteroids remain effective in providing short-term pain relief, their potential complications, such as tendon weakening and limited use in athletes.On the other hand, PRP appears to offer more sustained improvements in shoulder function, which aligns with its growing use in musculoskeletal conditions. However, the findings of this study do not fully align with previous literature that has suggested PRP’s superiority in long-term outcomes and tendon healing. This discrepancy may be attributed to variations in study populations, PRP preparation techniques, and injection protocols, leading to high heterogeneity in the meta-analysis. Future studies should standardize PRP protocols, include longer follow-ups, and stratify patients by injury severity to optimize treatment. Assessing PRP’s cost-effectiveness and role in therapy will further guide clinical practice.