INTRODUCTION
Total knee arthroplasty (TKA) is a well-established and widely performed reconstructive procedure designed to alleviate pain and restore function in patients with end-stage degenerative joint disease of the knee, most commonly due to osteoarthritis. The procedure involves resection of the distal femur and proximal tibia, with or without patellar resurfacing, and implantation of a prosthetic joint designed to mimic the kinematics of the native knee. Over the past several decades, advances in prosthetic design, surgical technique, perioperative optimization, and postoperative rehabilitation have resulted in improved outcomes and patient satisfaction, with survival rates of modern implants exceeding 90% at 10 to 15 years postoperatively in appropriately selected patients.1
As the aging population expands and the demand for improved mobility and quality of life increases, the utilization of TKA continues to rise globally. In the United States alone, more than 700,000 primary TKAs are performed annually,2 and this number is expected to increase 143% by 2050.3,4 The procedure is indicated for patients with symptomatic, radiographically confirmed joint disease that has failed conservative management, including physical therapy, pharmacologic interventions, and intra-articular injections. Key objectives of the procedure include restoration of mechanical alignment, preservation of joint stability, and optimization of implant fixation and longevity. Enhanced recovery after surgery (ERAS) protocols,5,6 regional anesthesia techniques,7 and multimodal analgesia regimens8 have further contributed to the safety and efficiency of TKA, allowing for shorter hospital stays and reduced complication rates.
Despite these advances, TKA remains a major orthopedic intervention with inherent surgical and medical risks. Among the most serious and potentially fatal perioperative complications is venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE). While much attention has been directed toward postoperative DVT, pulmonary embolism—due to its sudden onset and risk of hemodynamic instability—remains one of the most concerning complications following TKA and warrants continued surveillance and preventive strategies within the surgical community.
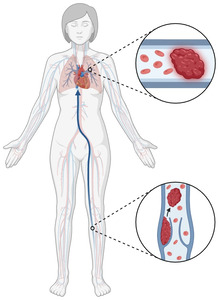
Pulmonary embolism occurs when thrombotic material, typically originating from the deep venous system of the lower extremities or pelvis, embolizes to the pulmonary vasculature, resulting in varying degrees of ventilation-perfusion mismatch, hypoxemia, right ventricular strain, and, in severe cases, cardiovascular collapse [Figure 1]. The pathogenesis of PE in the setting of TKA is multifactorial and closely aligned with Virchow’s triad: venous stasis, endothelial injury, and hypercoagulability.9
Orthopedic surgical procedures, particularly those involving the lower extremities, inherently increase the risk of thromboembolic events. Tourniquet application during TKA contributes to transient venous stasis and endothelial trauma, creating a milieu conducive to thrombus formation. For this reason, the use of a tourniquets for simple elective TKA is debated, although the practice is still widespread.10,11 Furthermore, manipulation of the lower extremity during surgery can dislodge existing thrombi or stimulate coagulation cascades via mechanical and inflammatory pathways. Postoperatively, reduced mobility, impaired venous return, and the hypercoagulable state induced by the surgical stress response collectively amplify the risk of thrombus propagation and embolization.
In this study, the authors examine a large national database to decipher predictors of pulmonary embolism following total knee arthroplasty.
METHODS
Data from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database were queried to examine venous thromboembolic peri/post-operative complications in patients undergoing total knee arthroplasty (TKA). The ACS NSQIP is a nationally validated, risk-adjusted, outcomes-based program designed to measure and improve the quality of surgical care. It collects over 150 variables, including preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes, across more than 700 participating hospitals in the United States. The reliability and accuracy of the NSQIP dataset have been extensively validated, with trained surgical clinical reviewers abstracting data using standardized definitions and protocols.12
Study Population
We queried the NSQIP Participant Use File (PUF) for the years 2018to 2022 to identify adult patients (age ≥18 years) who underwent total knee arthroplasty. TKA procedures were identified using Current Procedural Terminology (CPT) code 27447.
Variables and Definitions
The primary outcomes of interest included 30-day postoperative venous thromboembolism complications including pulmonary embolism (PE), and deep vein thrombosis (DVT) requiring treatment. These outcomes are systematically recorded in NSQIP according to standardized definitions and time frames.
Preoperative variables included age, sex, body mass index (BMI), smoking status, functional status (independent vs. not independent), American Society of Anesthesiologists (ASA) classification, and comorbidities including diabetes mellitus, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and steroid use.
Statistical Analysis
Descriptive statistics were used to summarize the baseline characteristics of the study population. Continuous variables were expressed as means with standard deviations or medians with interquartile ranges (IQR), depending on whether the data had a normal or skewed distribution. Categorical variables were expressed as frequencies and percentages.
Bivariate analyses were conducted to evaluate associations between baseline patient characteristics and postoperative outcomes using the Fisher’s exact test for categorical variables and t-tests or Mann–Whitney U tests for continuous variables, as appropriate. Multivariable logistic regression models were then constructed to identify independent predictors of adverse postoperative outcomes, adjusting for potential confounders. Covariates were selected a priori based on clinical relevance and previous literature and included age, sex, BMI, ASA classification, diabetes, smoking status, and operative time.
All statistical analyses were conducted using JMP version 17.0. Logistic regression analyses were performed to build models.13 Model discrimination was assessed using the area under the receiver operating characteristic (ROC) curve (AUC). Odds ratios (OR) with 95% confidence intervals (CIs) are reported for significant variables.14 Statistical significance was defined as a two-sided p-value <0.05.
Ethical Considerations
The use of the NSQIP database for this study meets the NIH criteria for exempt stduies, as it involves de-identified secondary data analysis. No patient identifiers were accessed or disclosed. The study complied with the Declaration of Helsinki and all applicable local and federal research regulations.
RESULTS
There were 150,119 TKAs performed during the study period, of which 61% were in women. The median age was 68 years with an interquartile range (IQR) of 61-74 years, and a range from 18 to 84. The median BMI was 32.3 with an IQR of 28.3 to 36.9. Table 1 summarizes the co-morbidities of the cohort.
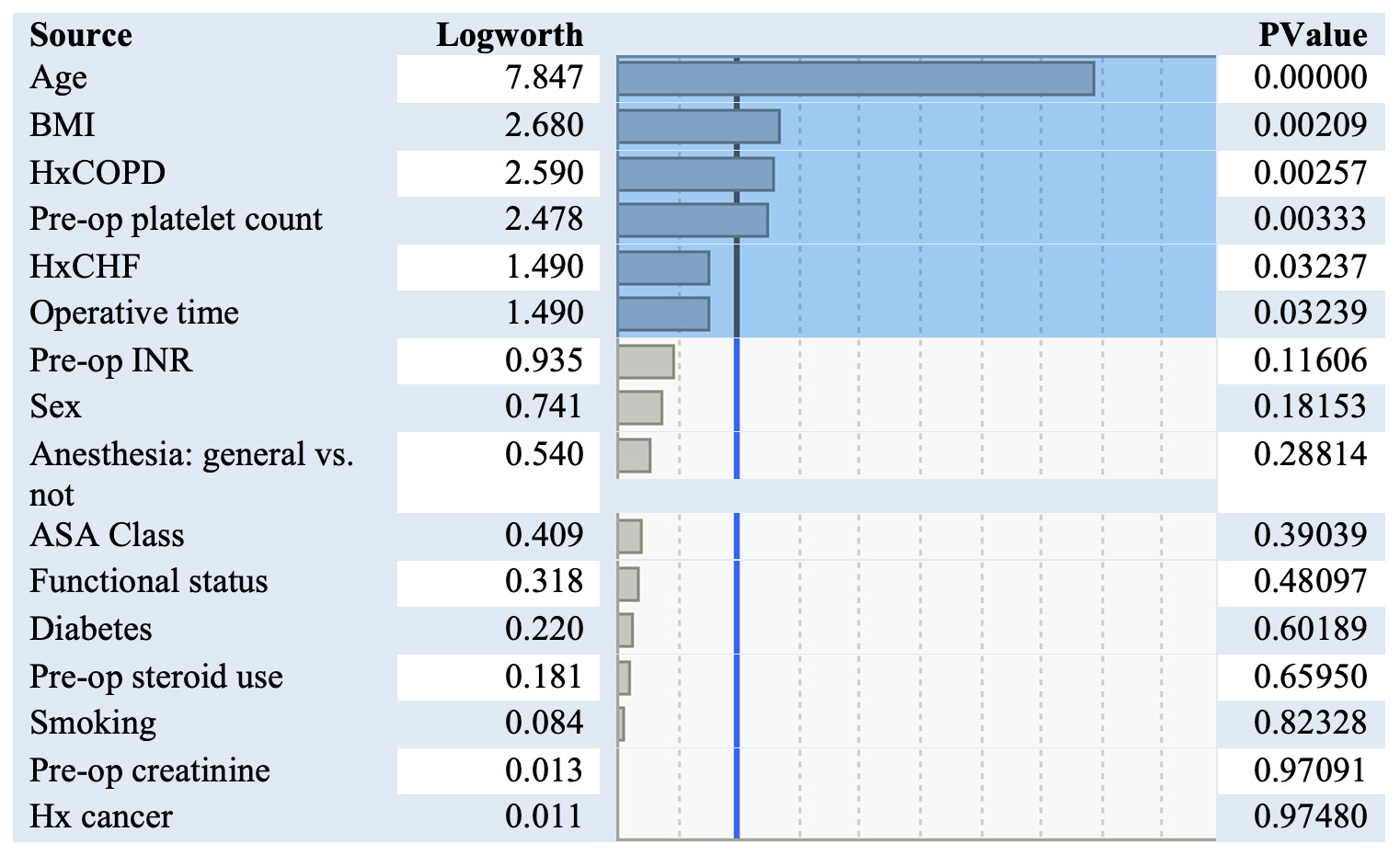
A total of 1114 patients had a PE (0.74%). The incidence was almost twice as high in women compared to men (756 vs. 387). The frequency of DVT for men and women were more similar (1093 for women, and 838 for men). Independent risk factors for pulomonary embolism are listed in figure 2. The variables most significantly associated with having a pulmonary embolism were older age, elevated BMI, a history of COPD or CHF, an elevated platelet count, and increased operative time.
For every one-year increase in age, the odds of having a PE increased by 2.9%. For every unit increase in BMI, the odds of having a PE increased by 1.9%. A history of CHF increased the odds of a postoperative PE by 180%, while a history of COPD increased the odds by 174%. Interestingly diabetes, which was much more prevalent of a comorbidity in the cohort, did not increase the odds of having a PE. Similarly, having cancer also did not increase the risk of PE, but the incidence was also very low. For every 10-minute increase in operative time, the odds of a PE increased by 2.2%.
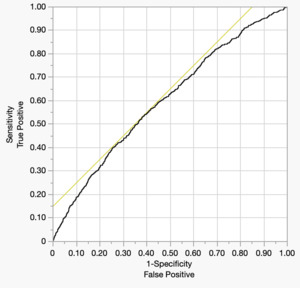
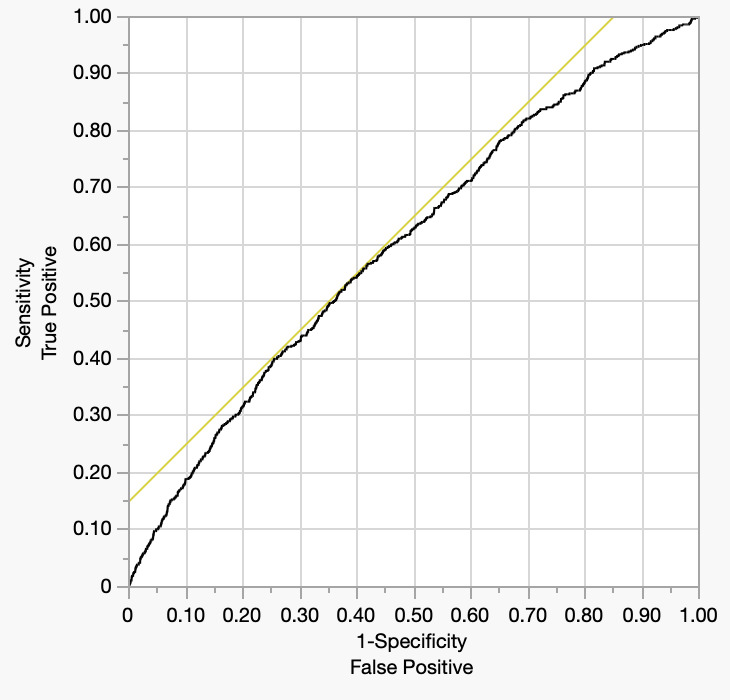
The Receiver operator characteristic is depicted below (Figure 3). The area under the curve is a modest 0.601.
DISCUSSION
Pulmonary embolism represents a leading cause of postoperative morbidity and mortality in patients undergoing TKA. Although its incidence has declined with the advent of standardized thromboprophylaxis protocols, symptomatic PE still occurs in approximately 0.3–1.5% of patients after TKA, with fatal PE reported in up to 0.1% of cases.15 Notably, these statistics may underestimate the true burden, as many embolic events remain clinically silent or are misattributed to other cardiopulmonary conditions. Moreover, the presentation of PE is often protean, ranging from subtle hypoxia and mild dyspnea to acute chest pain, syncope, and hemodynamic collapse, posing a diagnostic challenge in the immediate postoperative setting.
In the current study, we found that advanced age, elevated body mass index (BMI), chronic obstructive pulmonary disease (COPD), preoperative thrombocytosis or thrombocytopenia, congestive heart failure (CHF), and prolonged operative time are independently associated with increased odds of postoperative PE.
Age as a significant predictor of PE, aligns with prior studies that have consistently reported an age-dependent increase in venous thromboembolism (VTE) risk following orthopedic procedures.16 Aging is associated with diminished fibrinolytic activity, increased platelet aggregation, and reduced venous compliance, all of which contribute to a hypercoagulable milieu.17 Furthermore, older patients may have reduced mobility and greater comorbidity burden, both of which can delay postoperative ambulation and impair venous return. These physiologic and behavioral factors collectively increase susceptibility to thrombus formation and embolization in the early postoperative period.
Similarly, BMI was found to be positively correlated with PE risk. Obesity has been well-documented as a prothrombotic condition due to several mechanisms, including increased levels of plasminogen activator inhibitor-1, elevated inflammatory cytokines, impaired fibrinolysis, and mechanical venous stasis from increased intra-abdominal pressure.18 In the context of TKA, obese patients often experience technical challenges during surgery, reduced postoperative mobility, and altered pharmacokinetics of anticoagulant agents, which can further complicate VTE prophylaxis. Our findings add to the growing body of evidence supporting targeted interventions and weight management strategies in preoperative optimization programs.
The presence of chronic obstructive pulmonary disease (COPD) was also significantly associated with PE.19 Patients with COPD are known to have underlying pulmonary vascular remodeling and baseline hypoxemia, which may exacerbate the hemodynamic impact of embolic events. Additionally, the chronic inflammatory state of COPD may contribute to endothelial dysfunction and a prothrombotic state.20 In the postoperative period, impaired cough reflex, reduced ventilatory reserve, and difficulty with early mobilization may further compound the risk. Given these associations, heightened vigilance and possibly extended pharmacologic prophylaxis should be considered in this subgroup.
Congestive heart failure (CHF) emerged as another independent risk factor for PE, consistent with previous literature.21 CHF is associated with venous stasis, neurohormonal activation, and systemic inflammation, all of which promote a hypercoagulable state.22 Moreover, patients with CHF may have limited functional capacity, contributing to delayed mobilization and longer hospitalization—both recognized risk factors for VTE. The perioperative fluid shifts and hemodynamic fluctuations experienced by these patients may also potentiate right heart strain if a PE occurs, increasing the likelihood of clinically significant events. Our findings reinforce the need for careful fluid management, close monitoring, and potentially more aggressive thromboprophylaxis in patients with underlying cardiac dysfunction.
Lastly, operative time was a modifiable procedural factor significantly associated with PE development. Prolonged surgeries have been previously linked to higher VTE rates, potentially due to increased venous stasis, longer periods of anesthesia-induced immobility, and greater surgical tissue trauma.23 In TKA, extended operative duration may reflect technical difficulty, intraoperative complications, or bilateral procedures—all of which increase physiological stress and inflammation. From a systems perspective, reducing operative time through standardization of technique, use of experienced surgical teams, and preoperative planning may be a viable quality improvement target for minimizing thromboembolic risk.
The relatively modest discriminatory performance of the predictive model in this study, as evidenced by an area under the receiver operating characteristic (ROC) curve (AUC) of 0.61, suggests that the included clinical variables alone may be insufficient to fully capture the complex pathophysiology underlying postoperative pulmonary embolism (PE) following total knee arthroplasty (TKA). While factors such as age, BMI, COPD, CHF, operative time, and preoperative platelet count were identified as statistically significant predictors, these variables likely account for only a portion of the multifactorial risk associated with PE. Many important determinants of thromboembolic events—including genetic predisposition, perioperative hemodynamics, specific pharmacologic regimens (e.g., type and timing of anticoagulant used), and intraoperative events such as tourniquet duration or cement-related embolic phenomena—are not captured in the NSQIP database. Furthermore, patient-level variables such as medication adherence, timing of mobilization, and outpatient follow-up are also absent, limiting the model’s ability to fully stratify risk.
Another possible explanation for the modest AUC lies in the low incidence of clinically diagnosed PE in the postoperative TKA population. PE is a relatively rare outcome, occurring in approximately 0.3% to 1.5% of cases, and imbalanced class distributions in logistic regression models can attenuate predictive power. In such scenarios, even statistically significant associations may yield limited utility for clinical decision-making due to a low positive predictive value. Additionally, diagnostic misclassification may contribute to underdetection of PE events, particularly among patients with subtle or atypical symptoms that do not prompt confirmatory imaging. The NSQIP database, while rigorously curated, relies on clinical abstraction from operative notes and hospital records, which may not consistently capture subclinical or late-presenting embolic events. Taken together, these factors highlight the need for more granular, prospective data and possibly the inclusion of biomarkers or imaging findings to improve the predictive accuracy of models intended to identify patients at greatest risk for postoperative PE.
Collectively, these findings carry important implications for clinical practice. First, they support the development of personalized risk models that incorporate patient-level factors such as age, BMI, and comorbidities to guide the intensity and duration of thromboprophylaxis. While aspirin may suffice for low-risk patients, higher-risk individuals—such as those with CHF or COPD—may benefit from more potent anticoagulation strategies or extended prophylaxis beyond hospital discharge. Second, they highlight the value of multidisciplinary preoperative optimization programs, including cardiopulmonary evaluation, weight management, and hematologic screening, particularly in medically complex patients. Finally, they underscore the importance of operating room efficiency and adherence to enhanced recovery protocols to minimize modifiable procedural risks.
Limitations
This study has several strengths, including its large sample size, multicenter representation, and use of validated outcome definitions within the NSQIP framework. However, certain limitations merit discussion. The observational nature of the dataset limits causal inference, and unmeasured confounding may influence the observed associations. Additionally, NSQIP does not capture the timing, laterality, or severity of PE, nor does it account for the specific pharmacologic agents or mechanical methods used for thromboprophylaxis. Future studies incorporating longitudinal follow-up, imaging data, and detailed prophylaxis regimens would further elucidate the complex interplay between patient, surgical, and institutional factors in postoperative PE.
CONCLUSION
Our analysis identifies several clinically meaningful risk factors—advanced age, elevated BMI, COPD, abnormal platelet count, CHF, and prolonged operative time—that are independently associated with increased odds of pulmonary embolism following total knee arthroplasty. Recognition of these variables can inform preoperative counseling, enhance risk stratification, and guide personalized prophylaxis strategies aimed at reducing the incidence of this serious postoperative complication.