Background
Acute Diabetic Charcot Arthropathy (ADCA) is a rare, disabling condition that can cause widespread destruction of bone and joint architecture with loss of function. The pathogenesis of Charcot Arthropathy was classically described by French physician Jean Martin Charcot in 1883, but a complete understanding of this challenging condition continues to evolve. It is, however, certain that any condition resulting in loss of protective sensory innervation or autonomic neuropathy can lead to Charcot Arthropathy. Diabetes mellitus is currently the most common cause, typically affecting the foot due to the loss of its protective sensations.1
The inflammatory response elicited after unexpected injury to the ankle and foot can permanently disrupt the bony architecture of the foot, resulting in abnormal plantar pressures that increase the risk of ulceration, osteomyelitis, and amputation.2
The earlier stages of Charcot Arthropathy, often described as acute Charcot’s foot, remain a diagnostic challenge. It usually presents as a red, hot, swollen foot and may be indistinguishable from other causes of foot swelling such as cellulitis, sprains, or deep vein thrombosis.1
Correct diagnosis and treatment of acute Charcot Arthropathy are essential to decrease permanent foot deformity and allow for a stable, plantigrade foot that is suitable for ambulation.2
Pathogenesis of Acute Diabetic Charcot Arthropathy
Neurogenic Factors
Charcot Arthropathy has been documented to occur as a consequence of various peripheral neuropathies; however, diabetic neuropathy has become the most common etiology. The interaction of several component factors (diabetes, sensory-motor neuropathy, autonomic neuropathy, trauma, and metabolic abnormalities of bone) results in an acute localized inflammatory condition that may lead to varying degrees and patterns of bone destruction, subluxation, dislocation, and deformity.3
Loss of Sensory Innervation
In patients with sensory neuropathy, the inability to perceive pain prevents protective immobilization, allowing the inflammatory process to continue unchecked. This leads to a vicious cycle of inflammation and structural damage in the foot, exacerbating the condition. The release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) further drives inflammation, leading to osteolysis and progressive bone destruction.3
Impact of Sensory Neuropathy on Joint Stability
The loss of protective sensory feedback compromises joint stability. As the inflammatory response escalates, it leads to increased RANKL (Receptor Activator of Nuclear Factor Kappa-B Ligand) expression and osteoclast activation, which ultimately results in bone resorption and joint instability. Interestingly, while local inflammation is pronounced, there is often a dissociation from systemic inflammatory responses, highlighting the localized nature of the pathology.4
Theories Explaining Neurogenic Mechanisms
French Theory: This neuro-traumatic theory proposes that, in the presence of sensorimotor neuropathy, abnormal plantar pressure occurs due to muscle imbalance and loss of protective sensory feedback. This allows repetitive trauma that leads to microfractures and joint dislocation.5
German Theory: Conversely, the neurovascular theory suggests that autonomic neuropathy leads to a hyperemic state by increasing blood flow to the lower limbs through arteriovenous shunts. This hyperemia causes osteopenia, bone resorption, and weakening of the bone structure. Ultimately, it is on this weakened foot that spontaneous fractures and dislocations occur.5
Both theories provide insights into the mechanisms behind Charcot foot but do not fully account for its unilateral presentation, in contrast to the more common bilateral neuropathy seen in diabetic patients.5
Role of Peripheral Arterial Disease (PAD) in ADCA Progression
Peripheral arterial disease (PAD) plays a critical role in ADCA progression. Reduced blood flow impairs tissue oxygenation and healing, exacerbating inflammatory processes and promoting osteoclast activation. PAD in diabetic patients with Charcot arthropathy often leads to ischemic environments, which enhance bone resorption and increase the risk of foot deformities and amputations.6
Early detection of PAD through vascular assessments, such as ankle-brachial index (ABI) and toe-brachial index (TBI), is crucial to preventing further complications. Revascularization procedures, such as angioplasty, significantly improve outcomes in these patients by restoring adequate blood supply. Doppler spectrum analysis has proven to be a useful tool for evaluating disease activity in patients with Charcot neuroarthropathy. By monitoring blood flow in the first dorsal metatarsal artery, clinicians can assess the severity of inflammation and predict the appropriate time for weight-bearing or surgery. This method, as shown by Wu et al. indicates that a return to a triphasic Doppler pattern signifies reduced inflammation, providing a clear marker for disease stabilization.7
The prevalence of neuro-ischemic Charcot foot, where both peripheral arterial disease (PAD) and Charcot neuroarthropathy are present, is associated with a significantly worse prognosis. According to Meloni et al., neuro-ischemic Charcot foot patients experience higher rates of both minor and major amputations, as well as increased hospitalizations.8
These findings underscore the importance of early vascular intervention in improving outcomes for these patients.
Inflammatory Responses
A variety of causes are responsible for triggering, amplifying, and converting the inflammatory process. A markedly excessive local inflammatory response to trauma is known to be elicited in patients with acute Charcot’s foot. In contrast to the local inflammation, there is no systemic inflammatory response. As a result of local inflammation, pro-inflammatory cytokines—mainly tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β)—are produced excessively and beyond control.9
The physiological balance between the pro- and anti-inflammatory cytokines that restrains the inflammatory response to a necessary extent is then compromised.10
Mechanisms of Inflammation and Bone Resorption
TNF-α, IL-1β, and IL-6
The bone and soft tissues respond with an acute-phase release of pro-inflammatory cytokines: tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β). An increase in the amounts of TNF-α, IL-1β, and interleukin-6 (IL-6) has been found, whereas levels of interleukin-4 and interleukin-10, known as anti-inflammatory cytokines, were decreased. An abnormally intense and prolonged inflammatory response is inevitable under these circumstances.10
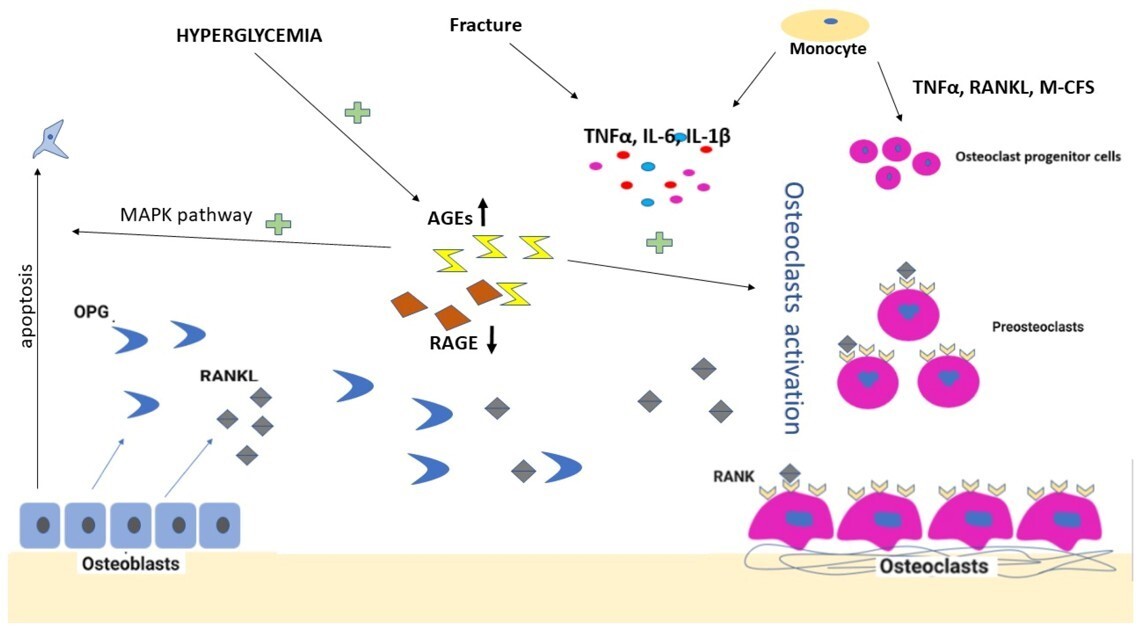
Increased amounts of pro-inflammatory cytokines, especially TNF-α, are found to be responsible for triggering another cytokine pathway that is centered on the polypeptide receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) (Fig. 1). As a member of the TNF superfamily, RANKL is the ligand that activates the receptor of NF-κB (RANK). The activation of RANK stimulates intracellular pathways that end up forming the nuclear transcription factor NF-κB. The expression of NF-κB induces osteoclast precursor cells to differentiate into mature osteoclasts (Fig. 1). Thus, the NF-κB pathway is implicated in the excessive osteoclastic activity in diabetic Charcot arthropathy, along with its involvement in many conditions that manifest with osteolysis, including glucocorticoid-induced osteoporosis, metastatic malignancy, periodontitis, prosthesis-related osteolysis, and rheumatoid arthritis.10
This can be useful as Monitoring local inflammatory markers, such as IL-6, can provide critical insights into the activity of Charcot neuroarthropathy. Jansen et al. found a significant arteriovenous flux of IL-6 in patients with acute Charcot foot, suggesting its role in both disease progression and as a potential target for therapeutic intervention.11
IL-17 Family
Despite the dominant role assigned to the pro-inflammatory cytokines belonging to the T-helper 1 and T-helper 2 cell (Th1/Th2) subsets of the immune system (e.g., IL-6, IL-1β, and TNF-α) as the driving force for the development and maintenance of inflammation-driven bone and joint diseases, it is now evident that Th1/Th2 cannot alone account for the pathogenesis of these disorders. Many inflammatory conditions are partly or largely driven by a new subset, namely the T-helper 17 (Th17) cells, which are the main source of IL-17 cytokines. This family is composed of six members ranging from IL-17A to IL-17F, which exert their biological actions through activation of several cascade systems, such as NF-κB and kinase signaling pathways. A growing number of studies in animals and humans have shown IL-17A to be pivotal in a multitude of bone-destructive processes, either by directly or indirectly stimulating osteoclastogenesis or by synergizing with other pro-inflammatory cytokines, such as TNF-α and IL-1β, in their bone-destructive role. Recent studies have shown that, despite being related, these cytokines have differential, non-overlapping, and sometimes opposing effects.12 (Fig. 1)
With increased understanding of the bone regulatory mechanisms controlled by members of the IL-17 family, results have shown a systemic increase of all three cytokines, suggesting that they take part in the bone repair and remodeling activity during the recovery phase of Charcot, as supported by studies showing direct T-cell involvement in the control of fracture healing. Additionally, the increase of IL-17A and IL-17E within a week after initiation of offloading treatment suggests that activation of these cytokines could have been triggered by fixation and immobilization of the foot, as fracture stability is a prerequisite for normal bone repair.12
Bone Remodeling Processes in Charcot Arthropathy
Osteomyelitis
Osteomyelitis is a well-documented complication in patients with Charcot arthropathy, especially among those with diabetes. This condition exacerbates the inflammatory response in the foot, complicating the acute phase of Charcot foot.14 The presence of osteomyelitis can lead to increased bone resorption and joint instability, necessitating vigilant management and intervention. Understanding the interplay between osteomyelitis and Charcot arthropathy is crucial, as early identification and treatment can mitigate further complications.
Imbalance Between Bone Formation and Resorption
A key feature of Charcot neuropathic arthropathy (CNA) is low bone mineral density, which is attributed to disordered bone turnover mediated through the RANKL-NFκB pathway.15,16 Research indicates that the acute phase of CNA is marked by upregulation of bone resorption, leading to bone tissue destruction without a compensatory increase in bone formation.17,18
This imbalance is critical, as it contributes significantly to the deformities and joint instability observed in affected patients (Fig. 2).
Role of Osteoclasts in Acute Charcot Arthropathy (ADCA)
Osteoclasts are pivotal in the pathogenesis of ADCA. Elevated RANKL levels have been linked to increased osteoclast activity, resulting in accelerated bone resorption in the affected areas.20,21 Additionally, pro-inflammatory cytokines such as TNF-α and IL-1β amplify this process, exacerbating structural damage in the foot.17,18 (Fig. 2) Understanding the role of osteoclasts can lead to targeted therapies aimed at modulating their activity (Fig. 2).
RANK/RANKL Pathway and Potential Therapeutic Targets
Low bone mineral density is a recognized characteristic of diabetes, particularly type 1, and this condition is further exacerbated in CNA, where disordered bone turnover occurs via the RANKL-NFκB pathway.22,23 The work of Gough et al. (1997) highlighted this by using biomarkers such as ICTP, PICP, and alkaline phosphatase to study bone turnover in CNA. They found a significant increase in ICTP in acute versus chronic CNA, with a strong correlation between local and systemic levels (r = 0.986) (Fig. 2). Importantly, no differences were observed in alkaline phosphatase or PICP levels, suggesting that acute CNA is characterized by increased bone resorption rather than decreased bone formation.
A small study comparing fracture and dislocation patterns in CNA revealed that decreased peripheral bone mineral density was associated only with the fracture pattern.24 This finding suggests that the type of deformity may depend on pre-existing bone density. RANKL levels are known to be upregulated by various pathological processes in diabetes, with increased glucose inducing NFκB activation and oxidative stress also playing a role.25 Immunohistochemistry has shown that RANKL is elevated in the serum of patients with CNA, highlighting its potential as a therapeutic target.26
Bergamini et al. (2016) presented a seemingly contradictory finding of decreased circulating RANKL in patients with acute CNA, suggesting a local compensatory mechanism that limits bone remodelling. Most studies have focused on chronic disease states, possibly providing different insights into RANKL expression.27
The Wnt/β-catenin signalling pathway is also disrupted in diabetes, with elevated serum levels of sclerostin and Dickkopf-1 noted in postmenopausal women with type 2 diabetes.28 Additionally, CD14-positive cells, which have a high potential for differentiation into osteoclasts, showed increased levels in CNA patients compared to those with diabetes and controls.29 This indicates that systemic factors and pro-inflammatory cytokines might contribute to the heightened bone resorption seen in CAN.30
A variety of interconnected processes appear responsible for the altered bone turnover in diabetic patients, including Wnt/β-catenin pathway disruption, increased circulating osteoclast precursors, and RANK/RANKL pathway activation. While the exact mechanisms remain unclear, they are likely influenced by elevated glucose levels and oxidative stress. Also, the receptor activator of nuclear factor kappa B ligand (RANKL)/osteoprotegerin (OPG) pathway is central to both bone resorption and vascular calcification in Charcot neuroarthropathy. Jeffcoate (2004) emphasizes that abnormal RANKL activation may contribute to both osteolysis and vascular calcification, particularly in diabetic patients with neuropathy.20 Targeting this pathway could help mitigate both the skeletal and vascular complications of Charcot foot.
Local Surgery of the Foot
In a case report from 2010 by J. Aragon-Sanchez et al., a patient who underwent conservative surgery for osteomyelitis subsequently developed acute Charcot in the midfoot. It was found that Charcot Arthropathy appeared to have been triggered by bone infection and/or surgery. It is believed that the pivotal factor in the development of acute Charcot neuroarthropathy in this case was the early weight-bearing on the deformed foot following the operation. Immobilization of the foot is critical, as it plays a significant role in decreasing the inflammation that contributes to the development of Charcot Arthropathy.31
When conservative treatment fails or deformity becomes severe, surgical intervention is necessary. The goals of surgery are to correct deformities, stabilize joints, and prevent ulceration.
Simultaneous Pancreas-Kidney Transplantation (SPKT)
A retrospective study conducted by Erika B. Rangel and colleagues found that 4.6% of recipients of simultaneous pancreas-kidney transplants (SPKT) were diagnosed with Charcot neuroarthropathy within the first year post-transplantation. The research indicated a significant association between cumulative glucocorticoid doses adjusted for body weight (exceeding 78 mg/kg) and unadjusted doses within the initial six months, with p-values of 0.001 and <0.0001, respectively, in relation to the onset of Charcot neuroarthropathy. The results suggest that glucocorticoids significantly contribute to the risk of developing Charcot neuroarthropathy after SPKT. The study recommends implementing protocols that either avoid or minimize glucocorticoid use to mitigate this risk.32
Current Research and Future Directions
Enhancing Diagnostic Accuracy and Early Recognition
Recent studies have emphasized that the acute phase of Charcot arthropathy is often misdiagnosed, leading to delays in appropriate management.33 The recognition of acute Charcot arthropathy as a potential first presentation of diabetes mellitus underscores the need for heightened awareness among healthcare providers.34
Understanding the Inflammatory Pathways
Inflammatory processes play a significant role in the acute phase of diabetic arthropathy. The presence of pro-inflammatory cytokines has been implicated in the pathophysiology of Charcot arthropathy, contributing to the inflammatory response observed in the affected joints (Fig. 3). This inflammatory response can lead to increased bone resorption and impaired healing, further exacerbating joint damage. The interplay between inflammation and neuropathy creates a complex environment that facilitates the transition from acute to chronic arthropathy.35
A better understanding of the molecular mechanisms that trigger and regulate bone repair in Charcot arthropathy is of particular relevance, especially when considering recent developments in the area of drugs targeting the Wnt and IL-17 signalling pathways, which hold great promise as future therapeutic approaches to treat common bone disorders.
Addressing Metabolic Dysregulation in Charcot Arthropathy
Metabolic dysregulation is another critical factor influencing the pathogenesis of acute diabetic arthropathy. Dysregulated glucose metabolism and the accumulation of advanced glycation end-products (AGEs) can adversely affect bone and joint health, leading to increased fragility and susceptibility to injury. Recent metabolomic studies have identified specific biomarkers associated with the risk of developing Charcot arthropathy, suggesting that metabolic profiling may provide insights into individual susceptibility and disease progression. For instance, certain amino acids have been linked to the incidence of Charcot arthropathy, indicating that metabolic pathways may play a role in the disease’s development.37
Research on Vascular Biomarkers and Advanced Therapies
Research on vascular biomarkers that predict PAD development in Charcot foot patients is an emerging area of interest. Identifying biomarkers early can enable timely revascularization, improving patient outcomes. Additionally, advances in gene therapy and regenerative medicine offer promising new avenues for treating the vascular complications associated with Charcot neuroarthropathy.38
Integrating Management of Comorbidities
Comorbidities such as obesity, chronic kidney disease, and peripheral vascular disease further complicate the pathogenesis of acute diabetic arthropathy. These conditions can exacerbate mechanical stress on the joints and impair glycemic control, increasing the risk of developing Charcot arthropathy. Additionally, the presence of diabetic foot ulcers significantly raises the risk of major complications, including amputation, highlighting the importance of comprehensive management strategies that address both diabetic control and foot health.39
Summary
ADCA remains a demanding challenge in the landscape of diabetic foot complications, known for its complex pathogenesis and potential for severe morbidity. This comprehensive review has shed light on several critical aspects of the disease.
Multifactorial Etiology
The pathogenesis of acute Charcot Arthropathy involves a detailed interplay between neuropathy, inflammatory responses, metabolic dysregulation, and comorbidities commonly associated with diabetes mellitus, making its management particularly challenging.
Staged Progression
Charcot Arthropathy typically progresses through distinct phases, each requiring specific management strategies. Early identification of the inflammatory stage is vital to prevent progression to chronic, irreversible deformities.
Treatment Challenges
Current treatment strategies, focused on offloading and orthotic interventions in the early stages, underscore the need for more advanced, targeted therapeutic approaches to halt disease progression.
Biomarkers and Neurotransmitters
The roles of inflammatory markers and neurotransmitters in the disease process remain incompletely understood. This presents significant opportunities for further research and the development of novel therapeutic targets.
Importance of Early Diagnosis
Early and accurate diagnosis by clinicians is essential in preventing the transition to chronic deformities and preserving foot and ankle function, which are crucial for maintaining mobility and quality of life in affected patients.
Future Directions
Several areas warrant further exploration based on the findings of this review:
-
Molecular Mechanisms: Deeper investigation into the molecular pathways that drive the initiation and progression of Charcot Arthropathy may reveal new therapeutic targets and improve understanding of the disease’s pathogenesis.
-
Immunomodulatory Therapies: The potential of immunobiological agents to modulate the inflammatory response during the acute phase requires exploration, offering new therapeutic possibilities for controlling disease activity.
-
Predictive Models: The development of predictive tools and risk assessment models could enhance early detection and enable personalized, targeted management strategies, improving patient outcomes.
-
Advanced Imaging Techniques: Investigation of novel imaging modalities could lead to earlier and more accurate diagnoses of Charcot Arthropathy, particularly in its pre-deformity stages, allowing for timely intervention.
-
Regenerative Medicine: Emerging fields such as stem cell therapy and tissue engineering offer promise for bone and joint regeneration in advanced cases, providing potential options for reversing or halting the progression of deformities.
Clinical Implications
The management of acute diabetic Charcot Arthropathy requires a comprehensive, multidisciplinary approach. Clinicians should:
-
Maintain a high index of suspicion for Charcot Arthropathy in diabetic patients presenting with foot and ankle symptoms, particularly in the presence of neuropathy.
-
Initiate prompt, aggressive interventions during the early inflammatory stage to prevent disease progression.
-
Employ a holistic treatment strategy that addresses the underlying neuropathy, inflammation, and metabolic factors contributing to the condition.
-
Strive to achieve a stable, plantigrade foot that is free of ulcerations and infections, allowing patients to maintain function and mobility.
-
Emphasize patient education, long-term follow-up, and preventative care to reduce the risk of recurrence and manage chronic complications effectively.
Conclusion
While substantial progress has been made in understanding the pathogenesis of acute diabetic Charcot Arthropathy, significant gaps remain. Continued research into the molecular mechanisms, coupled with advances in clinical practice, will pave the way for improved outcomes for patients affected by this challenging condition. As our understanding evolves, so will our ability to provide more effective, targeted interventions—ultimately enhancing the quality of life for individuals living with this complex manifestation of diabetic foot disease.
Country/Territory of origin
Egypt
Supplementary Materials
No supplementary materials are available for this manuscript.
Author Contributions
Conceptualization: Author 1, 2, 3, 4, 5, 6
Methodology: Author 1, 2, 3, 4, 5, 6
Writing – Original Draft Preparation: Author 1, 2
Writing – Review & Editing: Author 1, 2, 3, 4, 5, 6
Supervision: Author 3, 4, 5
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as it did not involve human or animal subjects.
Informed Consent Statement
Not applicable, as the study did not involve human participants.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to acknowledge Mansoura University Hospitals for providing resources and support during the course of this research.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Ethics Approval
Ethical approval was waived for this study as it is a narrative review of existing literature.
Consent for Publication
The authors give their consent for the publication of this manuscript. We confirm that the content has not been published or submitted for publication elsewhere, and that all authors have read and approved the final manuscript.