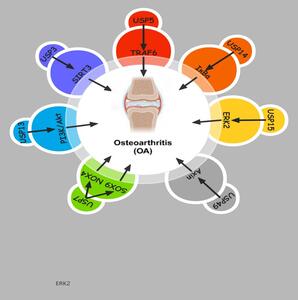
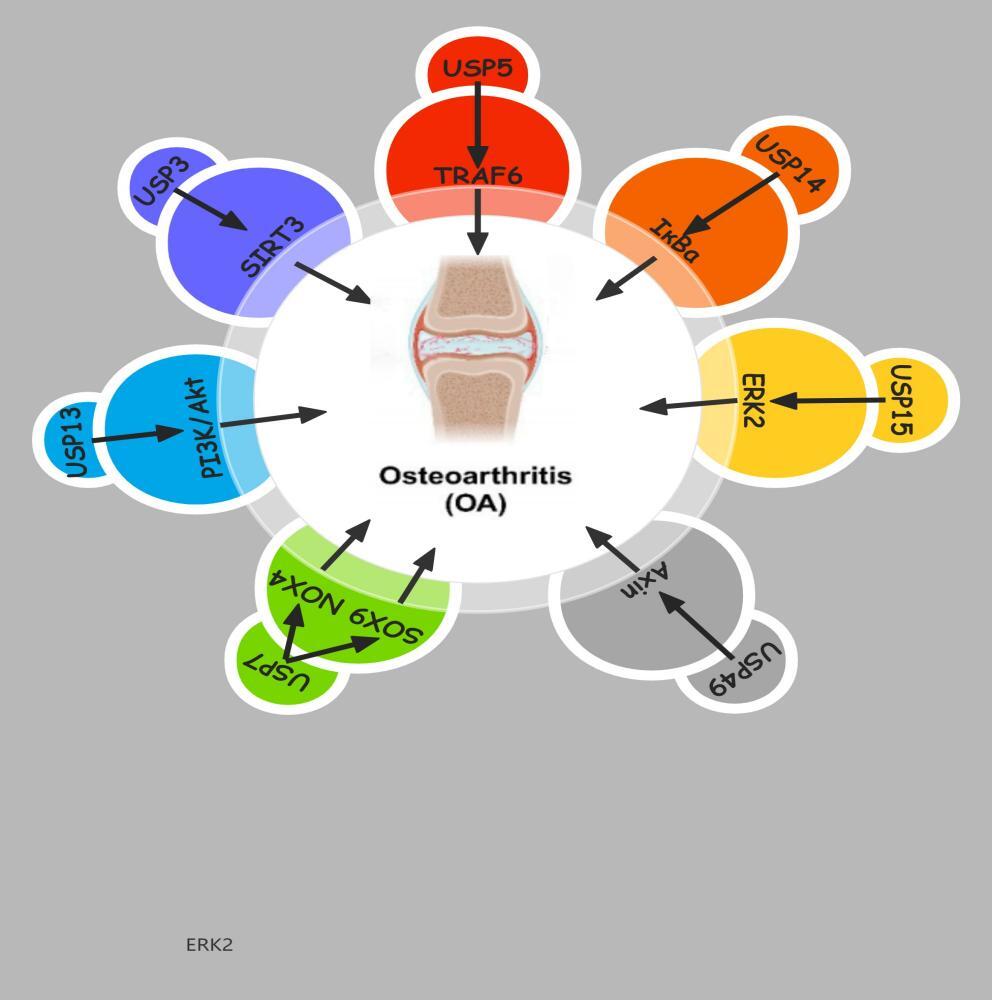
Highlights
-
Role of DUBs in OA Pathogenesis: Deubiquitinating enzymes (DUBs) are identified as key regulators in osteoarthritis (OA) progression, influencing cellular signaling, inflammation, apoptosis, and extracellular matrix degradation through the ubiquitin-proteasome system.
-
Therapeutic Potential: Targeting DUBs offers a novel therapeutic strategy for OA management. Small-molecule inhibitors of DUBs, such as USP7 inhibitors, show promise in reducing cartilage degradation and inflammatory responses in preclinical models.
-
Challenges and Future Directions: Current research on DUBs in OA is still in its infancy, with limitations in clinical validation and understanding of their complex interactions. Future studies should focus on elucidating the specific mechanisms of DUBs in OA and developing selective, safe, and effective DUB inhibitors for clinical use.
-
Clinical Implications: This review provides a comprehensive overview of the involvement of DUBs in OA, emphasizing their potential as therapeutic targets and the need for further research to translate these findings into effective treatments for OA patients.
1. INTRODUCTION
OA, a progressive joint disorder, is distinguished by the deterioration of cartilage within joints and is accompanied by joint pain, swelling, tenderness, and a decrease in mobility.1,2 As the population ages, the incidence of OA is gradually increasing, becoming an important public health issue.3,4 Although research on OA has increased in recent years, its exact pathogenesis is still not fully understood, which limits the development of effective treatment strategies for the disease.5,6 A variety of cytokines, enzymes, and signaling pathways are involved in the development of OA, among which the ubiquitin−proteasome system plays a significant role.6,7 Inside the cell, the ubiquitin−proteasome system is an important regulatory mechanism for protein degradation, with ubiquitination marking proteins for degradation. Correspondingly, deubiquitinating enzymes (DUBs) regulate protein stability, activity, and localization by removing ubiquitin chains from proteins. This process is essential for maintaining cellular homeostasis.8 In recent years, increasing evidence has demonstrated that aberrant gene expression or dysfunction of deubiquitinating enzymes is intricately connected to various diseases, including inflammatory diseases and degenerative joint diseases.9–11 Due to the key role of DUBs in regulating cellular signaling, inflammatory responses, and extracellular matrix (ECM) degradation, which are processes related to OA, they have emerged as targets in OA therapeutic research.12
Early studies focused on the role of deubiquitinating enzymes in cancer. However, as a deeper understanding of the function of the ubiquitin system develops, researchers have gradually begun to explore the role of DUBs in other complex diseases. In the mid-2000s, scientists first discovered the connection between DUBs and inflammation-related signaling pathways, and subsequent extensive research was conducted in the field of osteoarthritis. In recent years, increasing research has focused on specific DUB subtypes, such as USP and CYLD, which have been found to participate in the regulation of chondrocyte differentiation, extracellular matrix degradation, and the control of inflammatory responses.
Research on DUBs in osteoarthritis has gradually narrowed from the overall regulation of signaling pathways to the functional validation of specific enzymes, especially specific DUBs such as USP7 and USP15, which have been found to alleviate joint degeneration by inhibiting chondrocyte apoptosis and reducing inflammatory responses. Currently, the development of DUB inhibitors has entered the preclinical research stage, showing their potential as targets for osteoarthritis treatment. In the future, as the functions of DUBs are further elucidated, precise regulation of these enzymes’ activity is expected to bring new treatment options for OA patients. However, research in this field is still in its infancy, and more basic research and clinical trials are needed to verify the safety and efficacy of these potential therapeutic strategies. In light of this, this article aims to review the current understanding of the mechanisms of action of deubiquitinating enzymes in osteoarthritis and how they affect pathological processes and to explore potential therapeutic strategies based on these findings. By revealing the specific roles of deubiquitinating enzymes in the pathogenesis of OA and exploring their feasibility and safety as potential therapeutic targets, we ultimately hope to promote the development of more effective treatment strategies for OA.
2. Overview of the Biological Functions of Deubiquitinating Enzymes
Deubiquitinating enzymes (DUBs) are a class of enzymes that can remove ubiquitin molecules from proteins, and their biological functions within the cell are crucial. Ubiquitination is a process that regulates the stability, activity, and localization of target proteins by covalently attaching ubiquitin to them. This mechanism plays an important role in cellular physiology, affecting protein degradation, signal transduction, and the cell cycle, among other processes. Meanwhile, DUBs regulate the fate of target proteins by removing ubiquitin tags, thus maintaining protein homeostasis within the cell.
Ubiquitination is mediated by a trio of essential enzymes: the E1 activating enzyme, the E2 conjugating enzyme, and the E3 ligase. Initially, the E1 enzyme catalyzes the activation of ubiquitin and its subsequent transfer to the E2 enzyme. Following this, the E2 enzyme collaborates with the E3 ligase to catalyze the transfer of ubiquitin from the E2 enzyme to target substrate proteins. This post-translational modification enables the protein to be identified and subsequently degraded by the proteasome. Conversely, deubiquitination, which entails the removal of ubiquitin from these substrate proteins, is executed by deubiquitinating enzymes (DUBs), effectively counteracting the effects of ubiquitination.13,14
DUBs primarily function through two mechanisms. Firstly, DUBs can specifically remove ubiquitin from target proteins, promoting protein stability and function. For example, USP7 (Ubiquitin-Specific Peptidase 7) can remove ubiquitin marks associated with apoptosis, inhibiting cell death. It is thus crucial for cell survival and proliferation, especially under stress conditions. It also maintains the stability of multiple substrates, including p53 and Mdm2, which is significant for the regulation of the cell cycle and apoptosis.
Secondly, DUBs play a significant role in regulating cellular signal transduction. Take CYLD (Cylindromatosis) as an example; this deubiquitinating enzyme can promote the nuclear transport of NF-κB complexes by removing ubiquitin marks, thereby activating the transcription of downstream genes. CYLD is also involved in regulating the activity of the Wnt signaling pathway, affecting cell proliferation and differentiation.15,16 This signal modulation is crucial for maintaining cellular physiology and responding to external stimuli.
Additionally, DUBs are closely associated with cellular stress responses. USP15 can remove ubiquitination modifications when cells are under stress, promoting autophagy and helping cells clear damaged proteins and organelles. Additionally, DUBs play a pivotal role in the modulation of diverse biological activities, including cellular migration and inflammatory reactions, further emphasizing their importance in cellular functions.
The function of DUBs is not only related to their catalytic activity but also influenced by the cellular environment, substrate specificity, and other regulatory factors. Abnormal expression or dysfunction of DUBs is associated with various diseases, including cancer, neurodegenerative diseases, and autoimmune diseases.17–20 Therefore, studying the biological functions of DUBs not only helps us understand their importance in cellular physiology but also provides potential targets for disease treatment.
3. The Mechanism of Action of Deubiquitinating Enzymes in Osteoarthritis
3.1. Deubiquitinating Enzymes and Cellular Signal Transduction
In the pathological process of osteoarthritis, deubiquitinating enzymes help regulate cellular signal transduction through multiple pathways, which play an important role in maintaining joint health and responding to pathological conditions. Deubiquitinating enzymes can regulate the stability and function of signaling molecules related to inflammatory responses, cell proliferation, differentiation, and apoptosis.
Research has demonstrated that in the pathological state of OA, the expression and activity of certain deubiquitinating enzymes change, which may affect the ubiquitination levels of their substrates and thereby regulate related signaling molecules and cellular responses. These alterations amplify joint inflammation, disturb cartilage homeostasis and tilt signaling networks toward catabolism, thereby accelerating OA progression.
In recent years, the association between deubiquitinating enzymes (DUBs) and cellular signal transduction has been widely studied. The diverse roles of DUBs in processes of cellular transformation, such as oncogenesis, cell cycle regulation, cell metabolism, inflammation, and cell death, are continuously revealed.21,22 In cancer, DUBs have been found to have significant impacts on the tumor microenvironment, cell cycle regulation, metabolic pathways, and drug resistance.23 DUBs also have an important function in inflammation and cell death signaling. Studies have shown that OTULIN and LUBAC act together in the TNF receptor signaling pathway, affecting inflammation and cell death signals.24 OTUB1 regulates the degradation of cIAP1 through deubiquitination, via inhibiting TNF-induced cell apoptosis, thus playing an important role in the regulation of inflammatory signals.25 At the same time, the OTU family of DUBs has attracted the attention of researchers due to its specific functions and regulatory mechanisms in signaling pathways. These DUBs regulate protein stability, immune signaling, and cell cycles by modulating the ubiquitination status of proteins.
Deubiquitinating enzymes (DUBs) play a key role in cellular signal transduction, affecting fundamental processes such as cell cycles, apoptosis, and inflammatory responses. Reyes-Turcu et al. summarized how DUBs dynamically regulate various signaling pathways through the recognition of ubiquitin chain dynamics and emphasized their potential as targets in diseases. A20 is a classic DUB that regulates NF-κB signaling, and Catrysse et al. showed that it achieves negative feedback regulation of inflammatory signals through ubiquitin chain editing.26 In addition, DUBs are crucial for DNA repair, and Nijman et al. revealed that they maintain genomic stability by regulating ubiquitination.27 USP7 is closely linked to cancer development due to its influence on p53 homeostasis, and Cummins et al. pointed out that it may have value as a therapeutic target.28 Regarding antiviral immunity, Gack et al. found that the ubiquitination regulation of RIG-I achieves balance in activation and degradation.29 DUBs affect cellular signal transduction through various mechanisms and play a key role in multiple biological processes. Despite the considerable advances achieved in the field of DUB research, there remains a need to further elucidate the mechanisms that are specific to various diseases, the integration of upstream and downstream networks, and drug development.
3.2. Deubiquitinating Enzymes and the Cell Cycle
Deubiquitinating enzymes (DUBs) play a crucial role in the precise regulation of the cell cycle, affecting multiple processes: from DNA replication to centrosome replication to mitotic exit. Recent studies have revealed the importance of DUBs in maintaining cell cycle stability and genomic integrity, suggesting their potential as therapeutic targets.
DUBs dynamically regulate key ubiquitination pathway proteins. It has been found that USP7 regulates the stability of Mdm2 through deubiquitination, indirectly controlling p53 activity, and thereby affects G1 phase cell cycle arrest and DNA repair.30 Similarly, USP37 directly deubiquitinates Cyclin A, promoting the G1/S transition and participating in the regulation of DNA replication initiation licensing to prevent premature DNA replication.31,32 In addition, USP15 maintains the function of key proteins in the DNA replication process during the S phase checkpoint, ensuring the integrity of DNA replication and preventing genomic instability.33
DUBs also play an important role during mitosis. USP28 regulates the ubiquitination of Aurora kinase, affecting the exit from mitosis and preventing cell division errors.34 USP39 regulates the stability of proteins in the spindle checkpoint, ensuring the correct separation of chromosomes.35 Moreover, USP13 is involved in the regulation of centrosome replication, and its functional abnormalities may lead to polyploidy and cell cycle disorders.36 These studies indicate that DUBs play a crucial role in maintaining cellular homeostasis at multiple key points in the cell cycle.37
At the same time, certain DUBs further maintain the normal operation of the cell cycle by participating in DNA damage repair signaling pathways. It has been found that OTUB1 prevents the ubiquitination and degradation of DNA-damage-related proteins, preventing premature entry or arrest of the cell cycle, and thus protecting cells from genomic damage. In addition, USP7 and OTUB1 play an important protective role in DNA-damage-induced G1/S or G2/M checkpoints, providing a new perspective in the study of ubiquitin signal regulation during the DNA damage response.
Although the aforementioned studies have significantly improved the understanding how DUBs regulate the cell cycle, there remain some gaps in knowledge. First, most past studies have focused on a single DUB and its specific functions, without considering the global regulatory effects of the DUB network. Second, current research in this area is mainly completed in vitro, and so lacks validation for in vivo or in clinical models. Finally, the specific mechanisms of DUBs as potential therapeutic targets still need further exploration, especially regarding how specific DUBs can be selectively inhibited to avoid off-target effects.
3.3. Deubiquitinating Enzymes and Apoptosis
The role of deubiquitinating enzymes (DUBs) in apoptosis has gradually attracted the attention of researchers. Apoptosis—programmed cell death essential for development and immune regulation—also contributes to cartilage loss in OA. DUBs modulate this process by altering the ubiquitination status of key apoptotic regulators. DUBs affect the transmission of cell death signals by modulating the levels of protein ubiquitination, thereby playing an important role in apoptosis. Recent studies have revealed that multiple DUBs regulate apoptotic pathways through different mechanisms, including intervention in the ubiquitin−proteasome system and interactions with other intracellular signaling pathways.
One study summarized the multiple roles of DUBs in cell death, pointing out that DUBs not only play a role in apoptosis but are also involved in other forms of regulated cell death, such as necroptosis, pyroptosis, and ferroptosis.38 Another study explored the functions of DUBs in urinary system cancers, highlighting that DUBs can promote tumor cell survival by regulating apoptosis-related pathways and inhibit tumor development by activating apoptotic pathways.39
USP7 (also known as HAUSP) is an important DUB, and studies have shown that it regulates apoptosis by modulating the p53-MDM2 pathway. USP7 can remove ubiquitin modifications from p53, thereby stabilizing p53 and activating the apoptotic program and polyubiquitinated MCL-1 (an anti-apoptotic protein) to maintain cell survival. The elevated expression of this gene is closely linked to the initiation and progression of a range of malignancies. Another study found that this key protein regulates the balance between autophagy and apoptosis, further revealing the complex role of DUBs in cell life and death decisions.40
CYLD (Cylindromatosis DUB) has been proven to be involved in regulating the NF-κB signaling pathway and apoptosis. CYLD inhibits the activity of NF-κB through deubiquitination, thereby promoting apoptosis. In addition, UCH-L1 (Ubiquitin Carboxy-drolase L1) also plays a role in neurodegenerative diseases. It regulates the apoptosis process of neurons through deubiquitination and is closely linked to the onset of several conditions, including Alzheimer’s disease.
Regarding the therapeutic potential of DUBs, researchers have begun to focus on the development of small-molecule inhibitors. Although some DUBs have been confirmed, these studies are mostly focused on cancer cells or specific disease models and lack broad clinical validation. Moreover, although there are studies on some DUBs as potential targets, their mechanisms of action in different types of cells are not fully understood. Therefore, future research should pay more attention to the function of DUBs in various cellular contexts and across pathological conditions.
3.4. Deubiquitinating Enzymes and Cellular Metabolism
The role of deubiquitinating enzymes (DUBs) in the regulation of cellular metabolism has received widespread attention in recent years, due to these enzymes’ effect on glucose metabolism, lipid metabolism, oxidative stress responses, and cancer, among other processes and conditions. Cancer metabolism research has shown that DUBs promote cancer cell growth and metabolic adaptation by regulating protein stability and signal pathway activity. As such, they have been put forward as potential therapeutic targets. For example, the Machado–Joseph family of deubiquitinating enzymes plays a key role in tumor metabolism, regulating the PTEN and AKT/mTOR pathways and so affecting cancer cell proliferation and metabolic balance.41 USP14 supports the energy metabolism of cancer cells by stabilizing proteins related to mitochondrial function.42 In addition, USP7 has been proven to affect glucose metabolism by regulating key enzymes of glycolysis, maintaining the high metabolic level of cancer cells.43
Studies have shown that DUBs not only regulate tumor metabolism but also play a protective role in oxidative stress responses. Deubiquitinating enzymes can enhance metabolic homeostasis by alleviating oxidative stress, thereby improving cell survival capabilities. In lipid metabolism, DUBs support the membrane biosynthesis needs of tumor cells by regulating the ubiquitination of enzymes related to lipid synthesis and degradation.44 In addition, research on DUB complexes has further revealed their synergistic effects in multiple metabolic pathways, emphasizing their role as core nodes in the metabolic regulation network.
Although significant progress has been made in elucidating the role of DUBs in cellular metabolism, many mechanisms remain unclear. For example, there is preliminary evidence of USP7’s role in glucose metabolism, but the detailed mechanism by which it regulates the key enzymes of glycolysis still needs in-depth study.45 Similarly, the understanding of how USP14 influences mitochondrial function is mostly based on single-cell line studies, and the universality of the results needs to be validated.46
Further research should combine metabolomics, gene editing, and animal model experiments to reveal the multi-level functions of DUBs and assess their clinical potential as therapeutic targets.
The regulatory role of DUBs in cellular metabolism provides important insights for basic research and clinical applications. Research on their potential as therapeutic targets is still in its infancy. Future research should explore their fine regulatory mechanisms in normal cells and tumor metabolism through multidisciplinary cooperation to provide new strategies for disease treatment.
4. The Role of Various Deubiquitinating Enzymes in Osteoarthritis
4.1. USP3
USP3, a deubiquitinating enzyme, plays a key role in the regulation of inflammatory responses and aging in chondrocytes. According to literature reports, ubiquitin-specific peptidase 3 (USP3) can deubiquitinate through the RIG-I-like receptor pathway, thereby inhibiting type I interferon signal transduction and triggering antiviral immune responses.47
Compared with healthy controls, OA cartilage displays markedly reduced USP3 expression; restoring USP3 levels attenuates IL-1β-induced chondrocyte senescence.The enhancement of USP3’s function can alleviate chondrocyte apoptosis mediated by IL-1β and lead to the inhibition of the NF-kB signaling pathway.47 Tumor necrosis factor receptor-associated factor 6 (TRAF6), an adapter protein in the NF-kB signaling pathway, is central to immune and inflammatory responses. IL-1β promotes the ubiquitination of TRAF6, and USP3 alleviates the aging of chondrocytes by inhibiting the NF-κB signaling pathway induced by IL-1β and reducing the ubiquitination of TRAF6.48 In addition, the upregulation of USP3 also helps maintain the stability of SIRT3 protein, reducing cell damage caused by oxidative stress. This process involves inhibiting the ubiquitination of SIRT3, thereby maintaining its protein level. In turn, the inhibition of SIRT3 activity weakens the upregulation effect of USP3 in the aging of chondrocytes.49 Similarly, the upregulation of SIRT3 can counteract the production of reactive oxygen species (ROS) and cell aging caused by the absence of USP3. At the molecular mechanism level, SIRT3 partially reduces chondrocyte aging by deacetylating FOXO3.
4.2. USP5
According to existing research, USP5 plays a crucial role in the development of inflammatory responses. Specifically, USP5 regulates neuropathic and inflammatory pain by enhancing the function of CaV3.2 channels.50 Disrupting the interaction between USP5 and Cav3.2 has been proven to alleviate these two types of pai.51 In addition, a peptide that can penetrate cells and target the cUBP domain of USP5 has been found to effectively relieve neuropathic and inflammatory pain. In pain signal transduction, IL-1b, a key medium, modulates the communication between Cav3.2 and USP5.52 Experimental data also show that blocking the binding of USP5 and Cav3.2 can protect female mice from mechanical hyperalgesia caused by peripheral inflammation.53 Further research revealed that USP5 is closely associated with the pro-inflammatory function of RA-FLS.54 Rheumatoid arthritis (RA) is a prevalent chronic condition characterized by autoimmune-mediated inflammation, in which RA-FLS shows increased USP5 expression, while OA-FLS (osteoarthritis fibroblast-like synoviocytes) shows decreased USP5 expression. The stimulation of IL-1b leads to an increase in USP5 expression over time.55 The increased expression of USP5 significantly enhances the activity of the NF-kB signaling cascade, thereby stimulating the synthesis of pro-inflammatory cytokines. On the contrary, the inhibition of USP5 reduces the release of cytokines and inhibits the activation of NF-kB. USP5 can interact with TRAF6 and stabilize TRAF6 through deubiquitination, thereby maintaining its stability in RA-FLS and controlling the inflammatory process.56
4.3. USP7
Osteoarthritis research is exploring the mechanism of action of USP7. Studies have shown that hydrogen peroxide (H2O2) can induce the expression of USP7 in rat chondrocytes, and subsequently increase the level of reactive oxygen species (ROS), thereby inhibiting the proliferation of chondrocytes. The removal of USP7 can eliminate cell pyroptosis and ROS induction mediated by H2O2 and inactivate NLRP3 inflammasomes. In addition, the upregulation of USP7 enhances pyroptosis, IL-1β and IL-18 levels, MMP-1, MMP13, and the activation of NLRP3 inflammasomes in rat chondrocytes by increasing the level of ROS. USP7 interacts with NOX4 to promote its ubiquitination in rat chondrocytes. In patients with osteoarthritis, USP7 and NOX4 are highly expressed, and the inhibition of NOX4 can eliminate the functions of chondrocyte pyroptosis, ROS induction, and NLRP3 activation mediated by USP7. The USP7 inhibitor P22077 attenuates osteoarthritis progression in monosodium iodoacetate (MIA)-injected mice, indicating that USP7 promotes osteoarthritis by regulating the NOX4/ROS/NLRP3 pathway. In osteoarthritis, the ubiquitination of LKB1 increases the activation of the AMPK pathway, inhibits the NLRP3 inflammasome response, and blocks chondrocyte pyroptosis. Within the articular cartilage of mice afflicted with osteoarthritis, the expression of USP7 is reduced, and silencing USP7 with siRNA or its inhibitor can promote the proliferation of chondrocytes and accelerate chondrocyte apoptosis. USP7 reduces the inflammatory response in the inflammatory process.
The inhibitors of USP7 promote the destruction of cartilage in mice with osteoarthritis by activating the BiP-eIF2a-ATF4-CHOP pathway in endoplasmic reticulum stress (ERS) and promoting the NF-kB/p65 pathway. Some compounds, including QNZ and 4-PBA, as well as CHOPsinas, reduce the expression of USP7, which helps to suppress the proliferation of chondrocytes and promote cell death, while mitigating the inflammatory reaction triggered by TNF-α.57
ADMA promotes the instability of SOX9, which is mediated by DDAH1. In patients with osteoarthritis, DDAH1 expression is diminished. Conversely, the expression of DDAH1 is augmented. Mice with a global or chondrocyte-specific deletion of the DDAH1 gene, which functions as an ADMA hydrolase, exhibit a swift advancement of osteoarthritis. ADMA promotes the progression of osteoarthritis by inducing the degeneration and aging of chondrocytes and destroying ECM deposition. ADMA engages with SOX9 and USP7, safeguarding SOX9 from deubiquitination by USP7, which subsequently facilitates the degradation of SOX9. Consequently, the elevation of DDAH1 expression could suppress ADMA concentrations and modulate the USP7-dependent deubiquitination of SOX9, potentially offering a therapeutic approach for osteoarthritis management.58
4.4. USP13
USP13 expression inversely correlates with synovial inflammation severity in OA, suggesting its protective role in dampening excessive immune responses. In the synovial specimens of patients with rheumatoid arthritis (RA), the expression of USP13 increases, while under the stimulation of LPS, TNF-α, and IL-1β, the expression of USP13 in human fibroblast-like synoviocytes (H-FLSs) decreases, indicating that USP13 may play a key role in the regulation of inflammatory responses. USP13 inhibits pulmonary inflammation by stabilizing the anti-inflammatory receptor IL-1R8/Sigirr, a mechanism that also applies to osteoarthritis.59 USP13 also regulates PTEN by reducing oxidative stress, regulating apoptosis, and inflammation, thereby improving osteoarthritis. Oxidative stress and apoptosis are key factors in osteoarthritis that lead to chondrocyte damage and joint inflammation. USP13 plays a protective role by upregulating Nrf-2 and downregulating Caspase-3, indicating its potential therapeutic value in regulating oxidative stress and apoptosis.60
In addition, USP13 functions by binding to PTEN and regulating the activation of AKT. Induced by IL-1β or TNF-α, the increased expression of USP13 mitigates the inflammatory reactions in H-FLS. This modulation is likely due to the heightened PTEN activity, the reduced phosphorylation of AKT, and the dampened NF-κB signaling pathway.61 This finding reveals the important role of USP13 in regulating inflammatory responses, especially in the pathological process of osteoarthritis. An increase in the levels of USP13 is associated with the suppression of genes that are pivotal to osteoclast function and inhibits the occurrence of osteoclasts. In osteoarthritis, excessive activity of osteoclasts can lead to bone loss, exacerbating joint damage. USP13 may provide a new strategy for the treatment of osteoarthritis by regulating the occurrence of osteoclasts.
In a collagen-induced arthritis (CIA) mouse model, USP13 alleviated synovial hyperplasia, cartilage damage, inflammation, and bone loss. These in vivo experimental results further confirm the protective effect of USP13 in osteoarthritis, especially in reducing joint inflammation and protecting cartilage.
USP13 exerts a complex influence on the development of osteoarthritis. It significantly impacts the progression of osteoarthritis by regulating inflammatory responses, oxidative stress, apoptosis, and the occurrence of osteoclasts. The upregulation of USP13 may thus provide a new target for the treatment of osteoarthritis, especially to regulate inflammation and inhibit bone loss.
4.5. USP14
It is well known that USP14 can regulate protein degradation. Li et al. have shown that USP14 promotes the activation of the NF-kB signaling pathway and accelerates chondrocyte dedifferentiation induced by IL-1 by regulating the degradation of IkBa.62 USP14 is highly elevated in the articular cartilage of patients with osteoarthritis and in chondrocytes induced by il-1b. ACHP, an inhibitor of IKK-b, can inactivate the NF-kB pathway and eliminate the upregulation of USP14. In turn, USP14 enhances the deubiquitination of IkBa, promoting the activation of NF-kB. Inhibiting NF-kB can effectively eliminate the dedifferentiating effect of USP14 mediated by IL-1b on chondrocytes. Consequently, targeting USP14 could represent a promising therapeutic strategy for managing osteoarthritis.
Studies have found that USP14 promotes the activation of the NF-kB signaling pathway by affecting the degradation of IkBa, thereby accelerating the dedifferentiation process of chondrocytes induced by IL-1. In the articular cartilage of patients with osteoarthritis, the expression of USP14 is significantly increased, especially in chondrocytes induced by IL-1β.
Further mechanistic studies have shown that USP14 can enhance the deubiquitination of IkBa, thereby promoting the activation of NF-kB. This activation is crucial for chondrocyte dedifferentiation, and the NF-kB signaling cascade is closely linked to both the inflammatory reactions and the breakdown of chondrocytes. Therefore, inhibiting the activity of the NF-kB signaling pathway can effectively eliminate the effect of USP14 mediated by IL-1β on chondrocyte dedifferentiation. In terms of therapeutic strategy, ACHP, as an inhibitor of IKK-b, can inactivate the NF-kB pathway, thereby inhibiting the upregulating effect of USP14. This inhibitory effect has potential therapeutic value for controlling the progression of osteoarthritis.
4.6. USP15
The role of USP15, a deubiquitinating enzyme, is a subject of increasing interest in osteoarthritis (OA) research. USP15 is not only involved in various cellular processes and tumorigenesis but is also closely associated with the development of non-cancer diseases. In particular, USP15 plays a key role in the progression in osteoarthritis by finely regulating multiple signaling pathways through its deubiquitinating activity.
USP15 affects the activity of signaling pathways by regulating the deubiquitination status of target proteins, thereby playing a role in various diseases. In immune and inflammatory responses, USP15 is believed to be involved in modulating immune and inflammatory responses through the regulation of multiple signaling cascades, such as the TLR signaling pathways, the NF-kB pathway, the RIG-1 signaling pathway, the TGF-β pathway, and the p53 pathway.63 In particular, the role of USP15 in the TGF-β/SMAD2 signaling pathway is crucial for the pathogenesis of osteoarthritis. Studies have shown that USP15 can stimulate the TGF-β/SMAD2 signaling pathway, thereby affecting the phenotype and function of chondrocytes.
In the context of osteoarthritis, the interaction between USP15 and ERK2 is particularly important. ERK2 relies on USP15 to regulate the TGF-β/SMAD2 signaling pathway and control the phenotype of chondrocytes. At the molecular level, USP15 forms a complex with ERK2, which regulates the ubiquitination status of ERK2. USP15 activates the TGF-β/SMAD2 signaling pathway by increasing the level of phosphorylated-ERK1/2 but does not increase the stability of ERK2.64
The role of USP15 in osteoarthritis is multifaceted. It not only inhibits the progression of osteoarthritis by targeting the ERK and TGF-β/SMAD2 signaling pathways but may also affect the overall course of the disease by regulating immune and inflammatory responses. These discoveries offer novel therapeutic targets for the management of osteoarthritis; that is, by regulating the activity of USP15 or its interaction with ERK2, effective control of the progression of osteoarthritis may be achieved.
4.7. USP49
USP49, whose full name is ubiquitin-specific peptidase 49, is an enzyme that has received significant attention in the fields of oncology and osteoarthritis (OA). Research has demonstrated that USP49 is a crucial factor in the modulation of cellular proliferation, invasion, and programmed cell death.65 Individuals with osteoarthritis exhibit significantly lower USP49 expression compared to healthy individuals, which may be related to its role in chondrocyte apoptosis.
In patients with osteoarthritis, IL-1β, a key inflammatory factor, can activate primary chondrocytes derived from rats, suppressing USP49 protein expression. IL-1β may promote the degradation of the chondrocyte extracellular matrix, thereby affecting the integrity of articular cartilage. The downregulation of USP49 is closely related to chondrocyte apoptosis induced by IL-1β, which has been confirmed in vitro.66 In addition, the overexpression of USP49 can reduce chondrocyte apoptosis induced by IL-1β, and this effect may be related to the augmentation of Axin’s deubiquitination process. The increase in axin protein expression results in the subsequent breakdown of β-catenin, thereby suppressing the Wnt/β-catenin signaling pathway, which plays a pivotal role in the maintenance of chondrocyte viability and functionality.
5. Osteoarthritis Treatment Strategies Based on Deubiquitinating Enzymes
In recent years, research on deubiquitinating enzymes (DUBs) in osteoarthritis (OA) has attracted widespread attention. DUBs, by regulating ubiquitination modifications, influence cartilage homeostasis, inflammatory responses, apoptosis, and matrix degradation, providing potential targets for OA treatment.
Cartilage homeostasis is central to maintaining joint health. USP4 and CYLD play important roles in regulating chondrocyte survival and matrix metabolism, and studies have found that CYLD can significantly reduce cartilage damage by inhibiting ubiquitinated inflammatory signaling pathways, suggesting its potential as an ideal therapeutic target. Research on USP19 has emphasized its potential to promote chondrocyte repair by enhancing autophagy activity, highlighting autophagy pathway regulation as a new direction for treatment.67,68
Inflammation is an important pathological characteristic of OA. DUBs, such as USP15 and USP22, stand out for their ability to regulate inflammatory signaling pathways, and reduce synovial inflammation and cartilage damage by inhibiting the expression of NF-κB and related pro-inflammatory factors. In addition, USP7 has been recognized as a crucial element in regulating apoptosis, and its inhibitors can reduce the programmed cell death of chondrocytes, thereby delaying disease progression.
Oxidative stress is one of the key drivers of OA progression. The role of USP15 in antioxidant defense has been widely studied, and it has been shown to significantly alleviate oxidative damage both in vitro and in vivo.69,70At the same time, USP2 affects angiogenesis in diseased joints by regulating angiogenic factors such as VEGF. This provides a theoretical basis for anti-angiogenesis targeted therapy.
Matrix degradation is another significant feature of OA pathology, being mainly caused by the abnormal activation of matrix metalloproteinases (MMPs). USP5 directly regulates the ubiquitination degradation of MMPs to reduce their activity, thereby protecting the integrity of the cartilage matrix. In addition, the impact of USP10 on matrix stability has also been a point of focus, and its inhibitors have shown the potential to protect cartilage.
Osteophyte formation is a sign of late-stage OA changes. Research has demonstrated that USP21 is pivotal in modulating the development of osteophytes, and targeting its activity may effectively control the progression of osteoarticular changes. In addition, the regulatory mechanisms of synovitis and related pain signals have become new hotspots in DUB research, with CYLD and USP11 being considered as potential therapeutic targets.71,72
Although the existing research provides much theoretical support for the targeted application of DUBs in OA, some challenges remain. First, many previous studies have focused on in vitro experiments and animal models, and so lack sufficient clinical data. Second, the selectivity and long-term safety of DUB inhibitors still need to be optimized. In addition, the functional interactions between different DUBs and the clear mechanisms of specific targets require in-depth exploration.73
DUBs, as emerging targets for osteoarthritis treatment, show promising prospects for research and application. Future research should look to strengthen the understanding of their mechanisms and potential for drug development.74 In particular, multicenter clinical research is needed to achieve safer and more efficient targeted treatment strategies.
Several DUB-targeting inhibitors show promise in preclinical OA models: USP7 inhibitors (e.g., P22077 and P5091) attenuate cartilage degradation by suppressing the NOX4/ROS/NLRP3 axis and ER stress pathways (Fig. 1). P5091 reduces synovitis and chondrocyte pyroptosis in murine collagen-induced arthritis models. b-AP15 (targeting USP14/UCHL5) inhibits IL-1β-induced NF-κB activation and chondrocyte dedifferentiation, preserving ECM integrity. VLX1570 (USP14 inhibitor) mitigates oxidative stress and apoptosis in human OA chondrocytes.
Despite therapeutic potential, systemic DUB inhibition faces limitations: (1) On-target toxicity: USP7 inhibitors may disrupt p53-MDM2 homeostasis, causing hematological toxicity. (2) Off-target effects: b-AP15 concurrently inhibits proteasomal activity, leading to hepatotoxicity. (3) Pharmacokinetic hurdles: Low oral bioavailability and rapid clearance of DUB inhibitors (e.g., P5091) necessitate optimized formulations.
To overcome these challenges, targeted approaches are emerging: Intra-articular delivery: Liposome-encapsulated USP7 inhibitors (e.g., P22077) prolong joint retention and reduce systemic exposure in rat OA models. (2) Gene therapy: AAV-mediated overexpression of CYLD or USP13 in chondrocytes suppresses NF-κB and MMP-13 in murine OA, while shRNA knockdown of USP5 reduces synovitis. (3) Conditional activation: Prodrugs activated by cartilage-specific enzymes (e.g., MMP-13-cleavable USP14 inhibitors) are under exploration.
6. Discussion
6.1. Main Findings of the Study
This article summarizes and analyzes the latest research on the relationship between deubiquitinating enzymes and osteoarthritis (OA), related pathogenesis, and therapeutic strategies. As a class of important molecules in protein degradation and recycling, deubiquitinating enzymes serve as a crucial component in the occurrence and development of OA. Deubiquitinating enzymes may be important molecules affecting the pathological process of OAB, by modulating intracellular signaling pathways, apoptosis, and cellular metabolism (Figure 1).
Studies have shown that the mechanisms of action of deubiquitinating enzymes in OA are complex and diverse. They may affect the stability of the extracellular matrix, regulate the expression of cytokines, and participate in inflammatory responses through various pathways. In addition, abnormal activity of deubiquitinating enzymes may lead to an imbalance in cellular signal transduction, promoting the development of OA.
In response to the abnormal role of deubiquitinating enzymes in OA, researchers have proposed new therapeutic strategies based on these molecules. These strategies include the development of small-molecule inhibitors targeting specific deubiquitinating enzymes and the mitigation or reversal of OA progression by intervening in their activity. The development of these strategies will not only provide new targets for OA treatment but also open up new avenues of research.
However, our understanding of the function of deubiquitinating enzymes in OA and their use as therapeutic targets is still in its infancy. Future research needs to elucidate the specific mechanisms of these molecules, their interactions with other signaling molecules, and how these actions lead to the development of OA. In addition, clinically safe and effective drugs that intervene in the activity of deubiquitinating enzymes urgently need to be developed. Through in-depth research, we could not only comprehensively understand the pathophysiological mechanisms of OA but also achieve more effective disease prevention and treatment strategies.
6.2. Limitations of the Study and Future Research Directions
Although the connection between deubiquitinating enzymes and OA is recognized, the current understanding of their specific roles in this disease and how they interact with other molecular pathways to promote its progression remains limited. In addition, the regulation of deubiquitinating enzyme activity holds substantial importance for the identification of novel therapeutic targets, but how to achieve this, especially without affecting other important physiological processes, remains a challenge.
Future research directions include, but are not limited to, an in-depth exploration of the specific roles of deubiquitinating enzymes in the pathogenesis of OA to reveal how they regulate the activity of OA-related cytokines and proteases; confirming the impact of specific deubiquitinating enzyme inhibitors on OA progression through animal models and cell cultures; developing small-molecule drugs targeting specific deubiquitinating enzymes; and assessing drug safety and efficacy in preclinical settings. In addition, considering the multifactorial characteristics of OA, future research should also pay attention to the interactions between deubiquitinating enzymes and other molecules in the hope of finding more effective treatment strategies.
Acknowledgements
The researchers are supported by Dongguan Science and Technology of Social Development Program (No. 20231800940422,No. 20231800935592,No. 20231800935582).
Informed Consent Statement
Not applicable
Authors’ contributions
Zhikun Yuan: review concept and design; Yanhui Li and Zihui Zhao: drafting and revision, literature search and selection; Guanhao Chen design and revision; Haiyan Zhang: literature search and selection, data extraction. All authors read and approved the final manuscript.
Conflicts of interest
The authors do not believe that there is a conflict of interest that could potentially be construed to affect the material contained in the manuscript that is being submitted to the Journal.