INTRODUCTION
Pain after amputation is a symptom that often occurs in patients and can be categorized into residual limb pain and phantom limb pain. Phantom limb pain can be defined clinically as pain or discomfort in the no longer present extremity.1,2
A complex relationship between peripheral and central sensitization causes phantom limb pain. Several theories about the causes of phantom limb pain are currently being debated. The incidence of phantom limb pain is estimated to be related to the cause of amputation, diabetic/traumatic causes of amputation, pre-amputation pain, location of amputation, and treatment during amputation.1–3
In the US, 30,000 to 40,000 amputations are performed every year. It is estimated that in the US, by 2050, there will be 3.6 million people living without limbs. The primary causes leading to limb amputation include trauma, tumors, vascular disorders, and infections. Although the management of tumors prioritizes limb salvage, tumors that invade proximal nerves and vessels often render limb preservation impossible. This situation leaves radical amputation as the sole surgical option and the only chance for long-term survival. Tumors are responsible for 61% of upper limb amputations, with sarcomas accounting for a significant 72%.4–6 The incidence rate of phantom limb pain is diverse; the literature reports a low incidence rate of 33% to a high of 88%.3,7
Phantom limb pain after amputation is quite challenging to treat if it occurs. Current pharmacological and non-pharmacological studies show only minimal to moderate benefits. This will lead to a decrease in the patient’s quality of life and ongoing treatment burden. Therefore, a good prevention strategy is needed to reduce the incidence of phantom limb pain.1–3 The primary purpose of this systematic review is to examine ways to prevent phantom limb pain after amputation.
METHODS
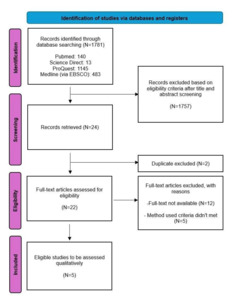
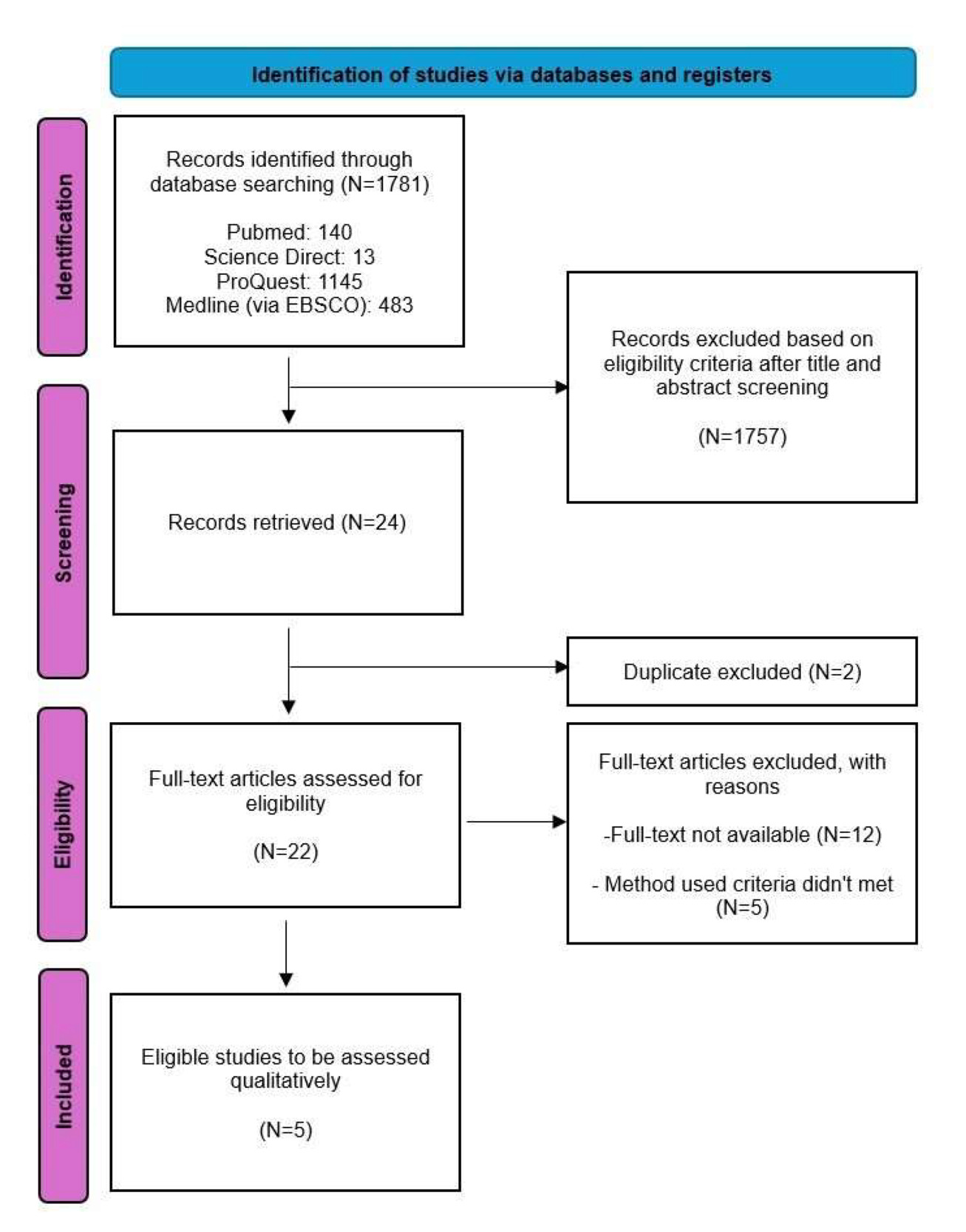
This study was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guideline [Figure 1]. A detailed protocol for this study has been previously registered in PROSPERO [ID: CRD42021284986].
Search strategy
A thorough literature search was performed on PubMed, Medline (via EBSCO), ProQuest, and Science Direct in January 2024 by two independent investigators using the keywords: ((prevention) OR (prophylactic)) AND (phantom limb pain).
Study selection
Human studies that measured the association between preventive methods for phantom limb pain were considered eligible. The inclusion criteria of the literature were (1) prevention/prophylaxis of phantom limb pain among amputees, (2) publication was done within the last 10 years, (3) randomized controlled trial or cohort study, and (4) written in English. Animal studies were excluded from this analysis.
Data extraction and quality assessment
Two independent reviewers performed data extraction and quality assessment. Different opinions between the two reviewers were resolved by reassessment and discussion with the third author.
The quality analysis of the literature was assessed with the Cochrane’s RoB for RCT8 and the Newcastle-Ottawa Scale for cohort studies.9 The Cochrane’s RoB is structured into a fixed set of domains of bias, focusing on different aspects of trial design, conduct, and reporting. In general, it involves five fundamental questions regarding bias that may occur in a research study, namely bias arising from the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. From these questions, a conclusion is drawn about the risk of bias in a study, categorized as low risk of bias, some concerns, or high risk of bias.10 New Ottawa Scale consists of nine questions. To evaluate the risk of bias, reviewers should rate each of the nine items as either present or absent. An overall score is calculated by adding the scores of all items. A score will be given for every paper to classify them as poor, fair, or good.9 The level of evidence was assessed using the Oxford Centre for Evidence-Based Medicine Guideline 2011.11
RESULTS
The characteristics of the five studies included in our study are shown in Table 1.
Study Selection
There were 1781 studies retrieved from 4 databases, namely PubMed, Science Direct, ProQuest, and Medline (via EBSCO). A total of 1746 studies were excluded after reviewing titles and abstracts. Of the 24 studies, two studies were excluded due to duplication. Of the remaining 22 studies, twelve were excluded because there was no full text, and five were excluded due to different methods.17–21
Study Characteristics
A total of 4 studies conducted by Purushotaman et al., Ayoub et al., Wang et al., and Yousef et al. were randomized controlled trials that were published in English. There was intervention before and after surgery in Ayoub’s investigation, and the onset of phantom limb pain was followed for six months.13 Wang intervened after surgery and was followed for 60 days.14 Purushotaman intervened for 7 days after the surgery, and follow-up was done for six months.12,15 Yousef used 100 IU of calcitonin as an additional substance in epidural anesthesia pre-operatively, and post-operatively on the first and second day. The patients were evaluated for one year. One study was conducted using the cohort method. The intervention was given at surgery, and was followed for six and eight months.16
Outcomes
Seven days of mirror therapy sessions, which were held for 20 minutes per session, were found to be effective in preventing phantom limb pain. Purushothaman et al. found that patients with mirror therapy tend to have a lower rate of developing phantom limb pain (11.7% vs 28.3%, p = 0.022). In patients who developed phantom limb pain, the intensity in 3 months was also found to be lower in the mirror group compared to the control group (p = 0.001).12 A randomized controlled trial held by Wang et al. in 2018 used gabapentin to prevent phantom limb pain among pediatric amputees. This showed a significant result in a lower incidence of phantom limb pain from the postoperative day until the last day of the visit (p = 0.033).14 Dexmedetomidine also showed a promising result in preventing phantom limb pain in an RCT held by Ayoub et al. in 2019. Dexmedetomidine was compared to fentanyl as an epidural infusion before and after the surgery to prevent phantom limb pain (p<0.05). In their study, Ayoub et al. found that the incidence of postoperative phantom pain in patients receiving dexmedetomidine in epidural infusion was significantly lower than in patients with fentanyl epidural infusion.13 According to a study by Yousef et al, patients who received epidural calcitonin experienced lower grade phantom pain throughout the follow-up period over one year (p = 0.044).15
A retrospective cohort study by Economides et al. found that the incidence of phantom pain was also significantly lower in the group of patients that underwent combined peripheral nerve coaptation, collagen nerve wrapping, and submuscular transposition, in the comparison group of patients that underwent traction neurectomy alone (p 0.03), the same result after six months (p 0.01).16
Quality Assessment
According to quality assessment, only one study was categorized as poor [Table 2 - 3]. The Oxford Centre for Evidence-Based Medicine Guideline 2011 evaluated the evidence level. Three studies had level 2 of evidence, and one study had level 3.
DISCUSSION
Phantom limb pain, which has an estimated prevalence of up to 80% among amputees, is an issue that can’t be ignored. Phantom limb pain might present with a sensation of neuropathic pain in the first week after limb ablation. This situation might lead to a decreased quality of life for amputees. Amputees have a lower quality of life related to daily activities, anxiety in young people, and depression in older people. These symptoms correlated with lower adherence in rehabilitation. Until now, no standard procedure has been used to prevent phantom limb pain.22–24 Our systematic review found five studies that can be used to prevent phantom limb pain in amputees. Among the five studies, one study was done in the surgical method, and four studies were done in the non-surgical procedure. Both methods showed promising results in preventing phantom limb pain.
Surgical Method
As a surgical method, Economides et al., in 2016, conducted a study to use peripheral nerve coaptation, collagen nerve wrapping, and submuscular transposition to prevent phantom limb pain in transfemoral limb ablation. Compared with traction neurectomy alone, peripheral nerve coaptation (common peroneal nerve to tibial nerve), collagen nerve wrapping, and submuscular transposition prevent phantom limb pain. After 2 and 6 months of follow-up, phantom limb pain is lower in the coaptation group than in the neurectomy group (0% vs 54.5%; p=0.03 and 0% vs 63.6%; p=0.01). Coaptation prevents nerve regeneration within surrounding tissue. The collagen nerve wrapping enhances repair and regeneration while reducing scar formation in surrounding tissue. Submuscular transpositions provide protection that reduces the risk of painful scar entrapment.16
Non-surgical Method
Non-surgical method, Ayoub et al.'s in 2019, compared dexmedetomidine (200 µg) to fentanyl (100 µg) as an epidural infusion 24 hours before surgery and 72 hours after surgery. The epidural infusion was stopped during surgery and continued 27 hours after surgery. The study showed that, compared to fentanyl, using dexmedetomidine as an adjuvant to epidural bupivacaine, 0.125% decreased phantom limb pain incidence. The visual analog scale was statistically significantly lower in dexmedetomidine than in fentanyl after the 72-hour postoperative period. Patient satisfaction and stump pain also showed a significant improvement in dexmedetomidine. However, follow-up after six months of surgery showed similar results (p < 0.05).13
With the same method, Yousef found that the addition of 100 IU of calcitonin to epidural infusion can significantly prevent severe phantom limb pain in a statistically significant manner. This outcome was observed at each follow-up at months 1, 3, 6, and 12 (p = 0.044). In the preoperative period, patients were given epidural injections using 1 mL of hyperbaric bupivacaine, followed by 10 mL of 0.5% bupivacaine, 100 micrograms of fentanyl, and 100 IU of calcitonin in the observational group. In comparison, the control group received 10 mL of 0.5% bupivacaine, 100 micrograms of fentanyl, and 1 mL of normal saline. On the first and second postoperative days, 10 mL of 0.5% bupivacaine and 100 IU of calcitonin were injected epidurally in the observational group, while 10 mL of 0.5% bupivacaine and 1 mL of normal saline were injected epidurally in the control group.15
Calcitonin has been explored as a treatment option for phantom limb pain, particularly in acute cases, but its efficacy for chronic PLP remains less clear. The analgesic effect of calcitonin is likely due to its action on both central and peripheral systems, involving serotonergic and catecholaminergic pathways, β-endorphin release, and calcium flux modulation.25 Furthermore, a systematic review by McConrmick Z. et al.26 indicated that perioperative calcitonin infusion may alleviate PLP severity over time, although results remain inconclusive. Calcitonin is considered relatively safe, and its potential for treating PLP warrants further research to establish optimal administration methods and long-term efficacy.
Dexmedetomidine is a new generation, highly selective α2-adrenergic receptor agonist. It inhibits the release of norepinephrine and subsequently decreases sympathetic tone through presynaptic activation of the 2-adrenoceptors, providing intense analgesia during the postoperative period. Dexmedetomidine also attenuates the hemodynamic and neuroendocrine responses to anesthesia and surgery, leading to sedation and analgesia.27–29
Dexmedetomidine can reduce the release of catecholamines at nerve endings by lowering sympathetic nervous system activity. The release of catecholamines is also inhibited directly through the action of 2 receptors on monoaminergic neurons. Thus, dexmedetomidine can reduce neuronal damage by inhibiting the release of neurotransmitters. It was also found that dexmedetomidine has a neuroprotective effect by inhibiting calcium ion influx and increasing glutamate release.14,28–30
Dexmedetomidine has also been found to be an alternative to fentanyl as an epidural adjuvant with comparable stable hemodynamics, early onset of sensory block, prolonged postoperative analgesia, lower consumption of local anesthetics for epidural analgesia in the postoperative period, and much better sedation levels.13,31
Adjuvant gabapentin therapy can also be used as a prevention method. Gabapentin is widely used as pharmacologic therapy for neurogenic pain. There are several hypothesized mechanisms of gabapentin in reducing pain, such as its role in increasing the release of gamma-aminobutyric acid, an inhibitory neurotransmitter, which may increase its inhibitory effect on pain neurotransmission. Studies have also shown that gabapentin can directly inhibit calcium channels by binding to the a2d-1 subunit, reducing presynaptic calcium intake and releasing excitatory neurotransmitters such as glutamate.14,32–34
The study by Wang et al. in 2018 used gabapentin as an adjuvant to limb ablation among pediatric patients. Gabapentin was given 300 mg on day 1, 600 mg on day 2, and 900 mg on day 3 until day 30 postoperative. The rate of phantom limb pain was significantly lower in the gabapentin group than in the placebo group (p = <0.05) at the last follow-up visit (43.48% vs. 77.27%, p = 0.033). Gabapentin has become increasingly popular as a treatment for neuropathic pain due to its efficacy, relatively mild side effects, and its use in cases of phantom limb pain. Gabapentin is effective in animal models of postoperative pain by providing a synergistic effect with a combination of opioids and NSAIDs.14
Non-operative treatment for phantom limb pain can also be conducted without the use of pharmacological medications, utilizing mirror therapy. Mirror therapy involves the reflection of voluntary movements in a mirror, performed by the intact limb, to restore its projection in the corresponding cortical motor and sensory areas. This process is believed to reduce pain associated with the disruption of sensory information.35,36 Mirror therapy has proven to be quite effective, as indicated by research conducted by Purushothaman et al. In their study, the mirror therapy group demonstrated a significantly lower incidence of phantom limb pain compared to the control group (11.7% vs. 28.3%; p = 0.022), along with lower Numeric Rating Scale (NRS) scores in patients experiencing phantom limb pain (4.5 vs. 5.6; p < 0.001).12
As some studies were pilot studies, a small number of studies with small sample sizes, ranging from 17 to 120 subjects, may limit our findings. With a small sample size, the result might differ if applied to a larger population. Hence, we suggest a more extensive study to build on existing research or to develop an innovative approach to prevent phantom limb pain, which can be a standard in its prevention.
CONCLUSION
Prevention of phantom limb pain can be done in terms of surgical and non-surgical approaches. Compared with neurectomy alone, peripheral nerve coaptation, collagen nerve wrapping, and submuscular transposition showed satisfactory results. The use of dexmedetomidine and calcitonin in epidural injection was also found to be beneficial in preventing the progression of phantom pain to a more severe degree when administered on the preoperative and the first two postoperative days. Oral gabapentin, as an adjuvant to limb ablation among pediatric patients, also reduces the incidence of phantom limb pain. Non-pharmacological therapy using mirror therapy also yields promising outcomes. These preventive approaches might reduce the incidence of phantom limb pain and improve the quality of life of amputees.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests
FUNDING
There is no funding for this study.
ACKNOWLEDGEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, M.P.J. and L.C.S.; Methodology, M.P.J. and L.C.S.; Investigation, J.A., M.A.U., A.D.P.S., and A.A.; Formal Analysis, J.A., and M.A.U.; Resources, A.D.P.S., and A.A.; Writing - Original Draft, M.P.J., L.C.S., and R.M.Y.; Writing - Review & Editing, L.C.S., and R.M.Y.; Visualization, M.P.J., Y.A.P.P., T.S.; Supervision, M.P.J., Y.A.P.P., T.S.; Funding Acquisition, M.P.J.
CLINICAL TRIAL NUMBER
Not applicable
HUMAN ETHICS AND CONSENT TO PARTICIPATE DECLARATIONS
Not applicable
CONSENT TO PUBLISH DECLARATION
Not applicable