Introduction
Osteoarthritis (OA) is a common degenerative joint disease, causing pain and functional limitation of elderly population. The primary osteoarthritis of the ankle is a very rare condition, while secondary OA of this joint is frequently posttraumatic or caused by inflammatory conditions. The rarity of primary form can be explained by anatomical difference: the surface area is small and the cartilage is thin.1 As etiology of ankle OA is mainly posttraumatic, it tends to affect the younger population of 18-44 years old.2 Treatment includes conservative therapy and surgical procedures, including joint-preserving and joint-sacrificing one. Conservative treatment is aimed on delaying the need in surgical intervention and includes non-pharmacological therapy as physical exercises, lifestyle change orthotics and pharmacological therapy being Nonsteroidal antiinflammatory drugs, intra-articular corticosteroid injections, viscosupplementation, platelet-rich plasma and mesenchymal stem cells. Although there are a variety of options, there is no consensus on the approach to treatment of patients with OA of the ankle joint.3
In recent years, hydrogels have gained attention as new biomaterials with therapeutic potential in cartilage repair and OA management. Hydrogels are hydrophilic, three-dimensional polymer networks that can mimic the viscoelastic properties of native articular cartilage. It can also serve as scaffolds, drug carriers, or stem cell delivery vehicles.4 The material used to prepare the hydrogels can be natural, including hyaluronic acid, chitosan, alginate, fibrin, collagen, elastin and synthetic including polyethylene glycol, polyglutamic acid based polymers.5 Their injectable nature allows for minimally invasive implementation, which is especially advantageous in joints with limited anatomical access such as the talus. There are numerous studies investigating the therapeutic features of hydrogel injection in knee OA, however its application in the ankle joint remains underexplored. The unique biomechanics of the ankle, which can be characterized by high congruence and load transmission through a relatively thin cartilage layer, makes the response to treatment different. Potentially it might affect the retention of hyaluronic acid within the joint. These biomechanical differences highlight the importance of ankle-specific research despite the rarity than adapting findings from the knee. Therefore it is necessary to analyze the response of the ankle to hydrogel-based treatment and evaluate long-term outcomes.
Given the potential of hydrogels to function as a joint-preserving treatment option, this systematic review considers its use in talus OA. This review aims to evaluate the effectiveness, safety, and clinical utility of hydrogel-based therapies for talocrural joint osteoarthritis, with a focus on identifying knowledge gaps and informing future research directions.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) checklist and the Cochrane Handbook for Systematic Reviews of Interventions.6,7
Literature Search Strategy
A comprehensive literature search was conducted in the first half of February 2025 up to the end of March 2025 across the electronic databases such as: PubMed, Scopus, Embase, and Google Scholar. The search was limited to publications in the English language. Following search phrases were used (“hydrogel” OR “injectable scaffold*” OR “biomaterial*” OR “viscosupplementation” OR “tissue engineering”) AND (talus OR ankle OR talocrural OR “ankle joint”) AND (osteoarthritis OR “cartilage degeneration” OR “joint disease” OR “articular cartilage”. The search was limited to studies from 2015 to ensure that latest outcome assessments were done, delivery techniques were up-to-date and there were advances in the formulation of HA.
Study Selection
There was a manual screening of titles and abstracts of publications by two independent reviewers. The articles which were unrelated to the focus were excluded. After the initial filter, all eligible studies were sorted alphabetically by first author and publication year. Disagreements between reviewers were resolved through discussion and, if necessary, arbitration by a third reviewer (YR).
Eligibility Criteria
Inclusion Criteria
Clinical studies such as randomized controlled studies, case series, cohort studies evaluating intra-articular injections of hydrogel-based therapies for ankle OA were eligible for inclusion to this review. Studies should have included quantitative outcomes on pain, joint functionality, imaging-based assessment. The article must be published in peer-reviewed journals.
Exclusion Criteria
Case reports were excluded. The studies investigating non-hydrogel-based therapies were excluded. The reports of in vitro, computational or cadaveric studies were excluded. The articles which are not focused on osteoarthritis (congenital defects) were excluded. Studies including stem cells without the hydrogel were excluded. Studies which did not include relevant quantitative outcomes were excluded.
Data Extraction
Two authors (DS and MM) independently extracted data from the included studies. Extracted variables included:
-
Study characteristics: author, year, journal, country, study design.
-
Population and intervention details: sample size, patient demographics, gender distribution, history of trauma, hydrogel composition, delivery method, follow-up duration.
-
Outcomes of interest:
-
Pain scales (e.g., Visual Analogue Scale (VAS)),
-
Functional scores (e.g., American Orthopaedic Foot & Ankle Society scale (AOFAS)),
-
Quality of life (if present)
-
Adverse events, complications, patient satisfaction and need for revision procedures.
-
All extracted data were cross-verified, and disagreements were discussed for consensus. The quality of randomized control trials (RCT) was evaluated by reviewing the adequacy of the randomization, completeness of the data presented.
Results
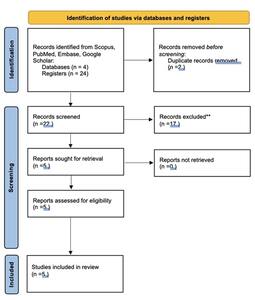
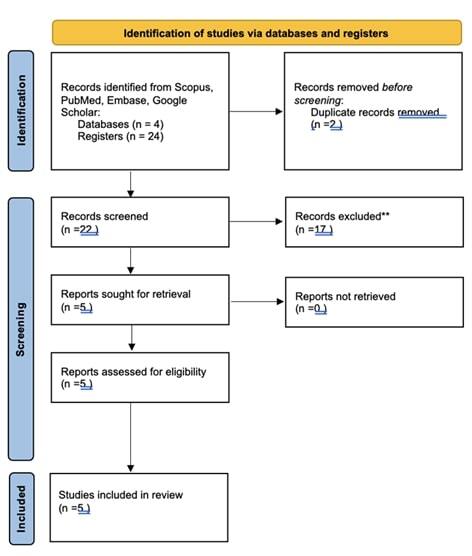
Primary search of the literature revealed 24 articles using PubMed and 0 articles using Embase according to the search of mentioned keywords. The abstracts were screened to exclude the papers published before 2014 (n= 14), non-hydrogel treatment (n=1), different joint (n=1) and different focus being surgical procedure (n=1). The search on Scopus revealed 1 duplication, which was excluded. The search on Google Scholar revealed 1 duplication. As reported in figure 1, a total of 5 studies were included in this review. The process of selection is reflected in Fig. 1.
As reported in Table 1, four out of five studies studies reported the effectiveness of Hyaluronic acid hydrogel. Interestingly, both single form and conjugated to another treatment modality revealed the effectiveness in terms of pain symptom reduction.
Study Characteristics and Interventions
A total of five studies met the inclusion criteria, studies conducted between 2019 and 2025. All studies evaluated intra-articular injection of hyaluronic acid (HA)-based hydrogels for talus OA. Of these, three studies included a placebo or comparative control group while two were single-arm cohort or non comparative trials.9–13
Hydrogel Types and Dosage
The types of HA investigated included non-animal stabilized HA with single injection approach,9 HA-CS with total 3 injections – 1 injection every week,10 DC-HA conjugate with 3 injections every 4 weeks for 12 weeks DC-HA conjugate with injection every 4 weeks for 1 year and HA-CS injections – 1 injection every week.11–13
Outcomes and Improvements
Four studies reported significant improvement in pain and functional scores. For instance, Younger et al. found a significant reduction in VAS pain scores and improved function. Gomez et al. and Woo et al. demonstrated that HA combined with CS resulted in greater and longer-lasting improvements compared to corticosteroid alone, as measured by AOFAS and VAS scales. Kubo et al. found no significant difference between DC-HA and placebo, while Nishida et al. observed symptom improvement over one year in a small cohort.
Safety and Complications
Younger et al. reported that most frequent adverse events reported by patients were arthraglia and headache (almost 25% each), nasopharyngitis (up to 20%) and pain in injection site ( 16%). Gomez et al. and Woo et al. reported no complications and adverse reactions. Kubo et al. reported common adverse events as common cold, injection site pain, nausea and palpirations in up to 7% cases. Nishida et al reported common adverse events as nasopharyngitis, eczema, injection site pain in more than 5% cases.
Discussion
This systematic review analysed existing publications on the effectiveness and safety of hydrogel-based therapies in the treatment of talus OA. According to inclusion criteria, five clinical studies were included in the review. The majority of them investigated HA-based hydrogels, either alone or in combination with other agents such as corticosteroids or nonsteroidal anti-inflammatory drugs (NSAIDs).
The findings of this review suggest that hyaluronic acid-based hydrogels are safe and provide symptomatic relief in patients with ankle OA. For example, study by Younger et al. and Woo et al. report improvement in pain score and joint function. Moreover, combination therapy with corticosteroid reported improved efficacy in comparison to corticosteroid alone and demonstrated prolonged symptom control.10 On the other hand, studies investigating HA combination with diclofenac revealed mixed results. According to Kubo et al., there is no superiority over the placebo, while Nishida et al. reported pain reduction within 1 year. This discrepancy can be explained by different regimen of the injections as in study by Kubo et al. there were 3 injections every 4 weeks for 12 weeks, while study by Nishida et al. reports injection every 4 weeks for 1 year. The longer administration might affect the reported outcomes. Moreover, study by Nishida is small, involving 8 patients only, therefore it is sensitive to variations such as age, joint condition, prior treatment history.
It was decided not to conduct the meta-analysis as there is a limited number of systematic reviews, heterogeneity in type of hydrogels, difference in control groups and protocols in term of number of injections. Moreover, there was a variety of the outcome measures reported across the studies. Therefore, authors decided to conduct the systematic review.
From previous studies, one large systematic review analyzed the effectiveness of hyaluronic acid by reviewing RCTs up to September 2014 and reported lack of data to claim efficacy or inefficacy as only 240 patients were included in the review. According to this study, the decrease in pain and physical function was reported by the participants. However, there was no evaluation in radiographic change and no data for the evaluation of the quality of life.14 Another systematic review evaluating the same period included 5 randomized controlled trials on use of hyaluronic acid intra-articular injections on 170 patients. The authors report improvement of functional scores and overall safety of the procedure, with no evidence of superiority of treatment option.15 The results of this review are in accordance with previous systematic reviews. However, the evidence remains preliminary, and current studies lack long-term follow-up and standardized outcome measures.
Limitations of the Current Literature
There are several limitations of this study that need to be taken into account when making final decisions. First is the relatively small sample size and short period of follow up (up to 12 months). It restricts from making generalisations of the findings. Next, is variability of outcome measures. Analytical comparison was not conducted as the AOFAS, VAS scores were used inconsistently and there is no uniformity in the result report. Moreover, there are different compositions of hydrogels used, which complicates the evaluation of efficacy. Studies rely on clinical symptoms, which imaging was not done in all studies and histological assessment was not conducted.
Additionally, no studies evaluated cartilage regeneration or integration using magnetic resonance imaging (MRI) or second-look arthroscopy, making it difficult to assess structural efficacy. Patient-specific factors, such as age, joint alignment, and history of trauma, were inconsistently reported and not analyzed as covariates.
One of the major limitations encountered during the review process was the fact that there are relatively few studies focusing on treating talus osteoarthritis using hydrogels. It was expected, as generally there is a lower incidence of ankle osteoarthritis. It is a challenge for collecting the data, which in turn creates the research gap. From this perspective, it is possible to consider studying the hydrogel across different joints to review the potential to regenerate the cartilage. It might provide a wider understanding of the biological effect of the hydrogel on the repair process. On the other hand, the biomechanical and anatomical differences of the joints must be considered too as there is different contact surface, thickness of the cartilage and load on the joint. Anyway, it would be rational to conduct comparative analysis across different joints.
Future Research Directions
The suggestions for further research are focused on addressing the limitations of the studies. So, it would be advantageous to conduct RCT with larger cohorts and longer follow ups. Moreover, there should be more standardisation of outcome reports (AOFAS, VAS, MRI-based scoring). Different formulations of the hydrogels might be used to evaluate the effectiveness of this therapeutic approach. For example, binding to different anti-inflammatory and proliferation stimulating biochemicals such as TGF-β or IGF-1 might be beneficial.16 Next, use of scaffold technique with different natural materials such as collagen or cartilage matrix might be considered.17 Moreover, the comparative analysis of use of hydrogel in osteoarthritis of different bones would provide an informative view of regenerative potential of hydrogels.
Conclusions
Hydrogels show potential for treating talus OA through cartilage support and symptom relief. However, the evidence base is limited, with a need for standardized outcome measures, larger trials, and joint-specific formulation research. Overall, while early evidence suggests that hydrogel-based therapies may offer a promising, joint-preserving approach for talus osteoarthritis, robust, long-term clinical trials are necessary to confirm efficacy and optimize hydrogel formulations for ankle-specific biomechanics.
Acknowledgments
We would like to thank Kanabekova Perizat for her invaluable assistance in researching and writing this article.
Authors’ contributions
Conceptualization: D.S., Y.R., B.B., A.B.; Data curation: M.M., B.S.; Formal analysis: D.S., M.M., T.T., A.B.; Investigation: D.S., M.M., Y.R., B.B.; Methodology: D.S., A.B., T.T., B.S.; Project admin-istration: D.S., Y.R., B.B.; Resources: M.M., T.T., B.S.; Software: A.B., T.T.; Supervision: D.S., Y.R., B.B., A.B.; Validation: M.M., B.S.; Visualization: D.S., M.M., T.T., B.S.; Writing – original draft: D.S., M.M., Y.R., B.B., A.B., T.T., B.S.; Writing – review & editing: D.S., M.M., T.T., B.S..
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP19679620). We would like to thank Kanabekova Perizat for her invaluable assistance in researching and writing this article.
Data Availability
All data analyzed in this systematic review were extracted from previously published studies, which are cited throughout the manuscript. No new data were generated.
Clinical trial number
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.