Introduction
The femur, the longest and strongest bone in the human body, forms a critical structural link between the hip and knee joints, playing an essential role in weight-bearing and locomotion.1 Traumatic femoral fractures most commonly result from high-energy mechanisms such as motor vehicle collisions, sports-related injuries, or falls from height. Standard surgical interventions include intramedullary nailing (IMN) and plate fixation.2–4 Femoral fracture repair is a relatively common orthopedic procedure, with an estimated annual incidence of 0.146–0.160% in the general population, translating to substantial healthcare utilization.5–7
Although surgical repair generally yields favorable outcomes, postoperative complications remain a significant concern, particularly in elderly populations where complication rates approach 35.8 Among these, thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), represent the most prominent adverse outcomes.9–11
Additional complications such as hemorrhage, infection, and fat embolism have been described; however, venous thromboembolism (VTE) is of particular concern due to its associated morbidity, mortality, and economic burden.12 VTE remains a leading postoperative complication following femoral fracture repair. Hospital costs associated with these events are considerable. A systematic review conducted in 2014 revealed that treatment of VTE was associated with incremental direct medical costs of $12,000 to $15,000 in 2014 US dollars.13 This study however was not specifically limited to postoperative VTEs. A 2020 study reported that perioperative DVT resulted in $22,301 more in hospital-related costs.14 A study specific to orthopedic surgery reported incremental costs ranging from $3,069 to $22,301 for DVT, approximately $6,604 for PE, and $51,576–$103,860 for VTE overall 15]. The economic burden of VTE persists beyond the acute postoperative period.15
Given these implications, it is critical to delineate patient- and surgery-related risk factors that predispose to thromboembolic complications. This study sought to identify perioperative predictors of VTE occurring within 30 days of femoral fracture repair, thereby informing preventative strategies and perioperative risk stratification.
Materials and Methods
Data were obtained from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), a robust national database encompassing over 11 million operations and more than 200 perioperative variables.
A total of 63,734 patients undergoing femoral fracture repair were included. Extracted data encompassed patient demographics, preoperative comorbidities, laboratory parameters, and surgical/anesthetic factors.16 Variables of interest included demographics (age, sex, body mass index [BMI]); preoperative risk status (ASA physical status classification II–IV), smoking, history of diabetes, congestive heart failure, and functional status (whether or not independent in activities of daily living); laboratory values such as preoperative international normalized ratio (INR), serum creatinine, and surgical factors such as operative time. Both univariate and multivariate logistic regression models were employed.17 Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to quantify associations between risk factors and 30-day postoperative thromboembolic events.18
This study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Participant Use File consisting of de-identified data. In accordance with federal guidance (45 CFR 46) and ACS-NSQIP data use policies, our institution (Brown University) reviewed the project and issued a Non-Human Subjects Research determination because no identifiable private information was used. Accordingly, formal IRB approval and informed consent were not required.
Results
Cohort Characteristics
The median operative time was 49 minutes, with an interquartile range of 35-70 minutes. There were 2110 patients with disseminated cancer, comprising 35 of the cohort. The median body mass index was 24.6, with an interquartile range from 21.3 to 28.4. The median INR was 1.1, with interquartile range 1.0 to 1.18.
Risk of deep venous Thrombosis
In the multivariable logistic regression model, disseminated cancer and operative time emerged as the strongest independent predictors of postoperative DVT following femoral fracture repair. Patients with disseminated cancer had a 65% higher risk of DVT compared to those without cancer (aOR 1.65, 95% CI 1.15–2.36, p=0.0065). Prolonged operative duration was also significantly associated with increased thrombotic risk; each additional minute of surgery conferred a 0.3% increase in DVT odds (aOR 1.003, 95% CI 1.002–1.005, p<0.001), corresponding to approximately 19% higher odds per 60 minutes and 31% per 90 minutes of operative time.
Body mass index demonstrated a borderline association with DVT, with each unit increase conferring a 1.4% increase in odds (aOR 1.014, 95% CI 1.00–1.03, p=0.056), though this did not meet conventional statistical significance. Higher ASA class was associated with thrombotic risk in global likelihood ratio testing (p=0.018), but no individual class-to-class comparison demonstrated significance after adjustment.
Other patient and perioperative variables, including age, sex, smoking status, diabetes, functional independence, dialysis, congestive heart failure, preoperative creatinine, platelet count, and international normalized ratio (INR), were not significantly associated with postoperative DVT in the adjusted model (all p>0.05).
The overall discriminative ability of the model was modest, with an area under the receiver operating characteristic curve (AUC) of 0.57, indicating limited ability to distinguish between patients with and without postoperative DVT.
Risk of Pulmonary embolism
Significant predictors in the multivariate model included length of operative time, the presence of disseminated cancer, higher preoperative INR values, and body mass index, as for risk of DVT. Additionally, for risk of PE a higher INR was protective [figure 1].
Operative Time (OPTIME)
Each additional operative minute increased the odds of PE by about 0.25% (aOR 1.002, 95% CI 1.001–1.004, p=0.012). Across the full range of operative times, this corresponded to more than a 30-fold increase in risk. This indicates that prolonged surgery substantially elevates thrombotic risk.
Patients with disseminated cancer had markedly increased odds of postoperative PE. The odds ratio was 2.77 (95% CI 1.92–4.00, p<0.0001), making this the strongest independent risk factor in the model.
International Normalized Ratio (PRINR)
Higher preoperative INR values were protective. The adjusted odds ratio was 0.43 (95% CI 0.23–0.80, p=0.008), suggesting that elevated INR (reflecting anticoagulation) significantly reduced the likelihood of DVT.
Body Mass Index (BMI)
BMI was an independent predictor, with each unit increase associated with a 1.8% rise in odds (aOR 1.02, 95% CI 1.00–1.04, p=0.031). Patients at the highest BMI values had nearly a fourfold higher risk compared to those at the lowest.
Other variables—including preoperative creatinine, platelet count, age, sex, ASA class, smoking, diabetes, functional status, dialysis, and congestive heart failure—did not independently predict postoperative DVT (all p>0.05). While some had point estimates suggestive of risk (e.g., dialysis OR ~1.99), the wide confidence intervals precluded statistical significance.
Model Performance
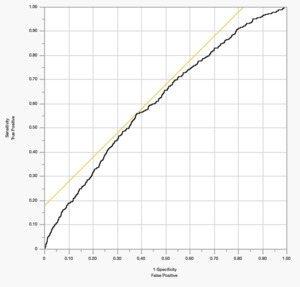
The discriminative ability of the model was modest, with an AUC of 0.61 [Figure 2], indicating limited ability to distinguish between patients who did and did not develop PE.19
Discussion
This study identified operative time and disseminated cancer as significant independent predictors of postoperative DVT following femoral fracture repair, with BMI showing a borderline effect. These findings are consistent with prior literature and carry important clinical implications.
Cancer patients represent a very high-risk subgroup requiring aggressive prevention strategies.
Attention to BMI and obesity-related risk may warrant inclusion in preoperative risk assessment, despite borderline statistical significance in this analysis.
The literature corroborates the central signals in our model—namely, the association of prolonged operative time and disseminated cancer with postoperative thrombosis—while also contextualizing the borderline effect observed for BMI. The largest cross-specialty analysis to date using American College of Surgeons NSQIP (1.43 million cases) demonstrated a stepwise rise in postoperative VTE across increasing surgical duration quintiles; patients in the longest duration stratum had 27% higher odds of VTE compared with average-length procedures after multivariable adjustment, a pattern that held for both DVT and PE and across surgical subspecialties. This seminal study by Kim and colleagues provides quantitative validation for operative time as an independent risk factor and supports our observation that even small per-minute effects accumulate to clinically meaningful risk over long procedures.20 While these data were from the 2005-2011 cohort, and thus precede the current analysis, the results remain robust.
Evidence from orthopedic trauma cohorts also supports time-and complexity-related risk. In a 56,299-patient NSQIP trauma study, Whiting et al. identified several independent predictors of DVT (e.g., ventilator use, steroid exposure, alcohol use), while confirming that lower-extremity injuries carry far greater risk than upper-extremity trauma; although the paper did not model anesthesia time directly, the authors emphasized markers of physiologic stress and case complexity that frequently track with longer operations. Our finding that operative time is independently associated with DVT therefore fits within a broader signal wherein markers of case severity and resource intensity portend higher thrombotic risk.21
Regarding malignancy, our results are concordant with the expansive cancer-associated thrombosis (CAT) literature. Khorana and colleagues’ work on CAT risk prediction (and the Khorana Score) underscores tumor-driven hypercoagulability, pro-inflammatory signaling, and treatment-related effects as key mechanisms; pooled data and narrative reviews consistently show higher perioperative and short-term VTE rates in patients with active or advanced cancer. Our observation that disseminated cancer independently elevates postoperative DVT risk is therefore expected biologically and clinically and is consistent with recommendations for intensified prophylaxis in high-risk oncology populations.22
The BMI signal in our model was borderline, which is not unprecedented. Meta-analytic and narrative reviews generally characterize obesity as a modest, independent VTE risk factor (often ~1.3–2.0×), with risk rising alongside BMI and age; however, the magnitude and statistical significance can attenuate after multivariable adjustment or in cohorts where obesity is common, narrowing variance. This heterogeneity is well described in contemporary syntheses and may explain our near-significant estimate—particularly if other correlated covariates (immobility, operative duration, transfusion) mediate part of the obesity effect23
Orthopedic studies focused on femoral shaft and lower-extremity fractures further illustrate context. Yang et al. reported a 26% preoperative DVT prevalence in isolated femoral shaft fractures, highlighting how injury-related stasis and inflammation can front-load thrombotic risk even before incision, while Ren et al. identified postoperative DVT risk factors after surgically treated femoral shaft fractures in a case–control design. These reports complement our perioperative findings by emphasizing that femoral-fracture patients may enter the OR with elevated baseline thrombotic burden, which then compounds with intraoperative exposures such as longer operative time.24
Several syntheses in orthopedic populations also align with our clinical implications. Systematic reviews of postoperative VTE consistently flag obesity, higher ASA class, immobility, and longer surgery as risk enhancers, and they advocate targeted or extended prophylaxis in selected high-risk groups—particularly after hip fracture or major lower-extremity procedures. The ACCP-aligned evidence base indicates that risk persists for weeks beyond discharge, supporting consideration of extended prophylaxis in patients who combine procedure-level risk (e.g., hip/femur surgery, prolonged operations) with patient-level factors (e.g., active cancer)25
Not all findings are universally consistent. Some orthopedic analyses find BMI loses significance once operative time, transfusion, or comorbidity indices are included; conversely, other series report significant BMI effects, especially at higher thresholds or in procedures with limited mobility postoperatively. This variability reinforces that BMI may function partly as a risk amplifier whose measurable effect size depends on cohort composition and modeling strategy23
Collectively, the external literature supports our central conclusions: prolonged operative exposure and disseminated cancer are reproducible and clinically meaningful DVT risk factors after femoral fracture repair, while BMI likely contributes a smaller, context-dependent effect. These data, together with the modest model discrimination in our analysis, argue for multifactorial risk stratification (combining patient, injury, and operative features) and for selective extension or intensification of prophylaxis in patients with converging risks
Evidence from orthopedic trauma cohorts also supports time-and complexity-related risk. In a 56,299-patient NSQIP trauma study, Whiting et al. identified several independent predictors of DVT (e.g., ventilator use, steroid exposure, alcohol use), while confirming that lower-extremity injuries carry far greater risk than upper-extremity trauma; although the paper did not model anesthesia time directly, the authors emphasized markers of physiologic stress and case complexity that frequently track with longer operations.21 Our finding that operative time is independently associated with DVT therefore fits within a broader signal wherein markers of case severity and resource intensity portend higher thrombotic risk.
The clinical implications of this study include several potential avenues for clinical action:
Patients undergoing prolonged operative procedures should be closely monitored and considered for extended thromboprophylaxis.
Limitations
This analysis is limited by its reliance on NSQIP variables, which do not capture all potential confounders (e.g., specific thromboprophylaxis regimens, mobility protocols). The model’s poor AUC reflects the need for additional predictors to enhance clinical utility.
Conclusion
Thromboembolic events remain a significant source of morbidity following femoral fracture repair. This NSQIP-based analysis identified underlying disseminated cancer, and longer operative time as predictors of venous thromboembolism, including deep venous thromboses and pulmonary emboli. These findings highlight the need for vigilant preoperative risk stratification and targeted prophylactic measures to improve outcomes in this vulnerable population.

_and_95__confidence_intervals_for_independent_predictors_o.png)
_and_95__confidence_intervals_for_independent_predictors_o.png)
