INTRODUCTION
Metatarsal fractures are one of the most common injuries involving the foot representing up to 6% of all the fractures seen in the primary care setting. Of these fractures, approximately 45–70% of these injuries involve the fifth metatarsal.1 These fractures affect a broad age distribution from athletic injuries sustained by younger patients to age-related falls.2–4 Patients commonly present with pain in the lateral aspect of the forefoot that is elevated with any weight-bearing activity. This pain may occur following an acute injury or after repetitive microtrauma induces injury over a chronic period. Most of these fractures involve the proximal region of the metatarsal (~70%) and in lower frequencies involve the shaft (17%).5,6 Proximal fractures are commonly classified into differing types and include three anatomical zones: Zone 1 (tuberosity), Zone 2 (metaphyseal-diaphyseal junction), and Zone 3 (diaphyseal area within 1.5 cm of the tuberosity). Zone 2 encompasses a vascular watershed with blood supply provided by metaphyseal and diaphyseal nutrient arteries.
A nonunion metatarsal fracture can cause pain, inflammation, deformity of the bone, and loss of functionality. Fifth metatarsal fractures have a high rate of nonunion (15-30%), especially in Zone 2 fractures. Fractures in Zone 2 have higher rates of nonunion when managed nonoperatively with estimates of 20-30%.6–8 In cases of nonunion, patients may experience recurring pain and disability along with increased utilization of healthcare resources (e.g., pain medication, rehabilitation, and surgery). Nonunion is associated with high complication rates and poor long-term outcomes.9,10 Treatment options for fifth metatarsal fractures typically involve rehabilitation therapies and may require operative intervention. Surgery is often performed on metatarsal fractures due to the risk of delayed healing or reinjury but may also result in nonunion.11
Pulsed electromagnetic field (PEMF) stimulation therapy is a modality that provides a non-surgical intervention to promote bone healing. This technology uses electromagnetic fields delivered to the targeted injury to produce beneficial effects at the cellular level that improve bone fracture healing. PEMF benefits include anti-inflammatory, regenerative, and protective cellular effects that aid in bone healing and functionality.12–14 PEMF therapy promotes the activity of osteoblasts and bone morphogenetic protein expression thereby enhancing osteogenesis and chondrocyte proliferation leading to improved bone formation and rates.15–18 PEMF treatment has been used in many musculoskeletal indications with positive effects including patients with cervical disc herniation, osteoarthritis, fibromyalgia, and osteoporosis.19–23
Due to the relatively high frequency of fifth metatarsal fractures and observed nonunion rates (specifically Zone II), it is critical to explore treatment options that target fracture healing and mitigate factors that contribute to poor outcomes. PEMF represents a treatment modality with known benefits and a favorable safety profile with the potential for high impact in this patient population. The current study aimed to evaluate real-world data on the utility of PEMF in patients with metatarsal fractures.
MATERIALS & METHODS
Study Design
The current study employed a multi-center, post-market, retrospective design to evaluate real-world performance of PEMF using stimulation devices (PhysioStim™ device, Orthofix US LLC, Lewisville, TX, USA) to treat metatarsal nonunion. Subjects were enrolled at five sites across the United States. Subjects were included in the study if they were diagnosed with metatarsal nonunion at the participating investigative site and eligible for treatment with an Orthofix PhysioStim device. No fractures were treated surgically. Subjects were enrolled who were 18 years or older at the time of treatment. Subjects were followed for up to 12 months after the start of treatment for metatarsal fracture nonunion to determine healing status which included a radiographic assessment. Subjects received standard of care follow-up, and therefore, there were no scheduled follow-up visits. Healing status was assessed radiographically using imaging with x-ray dorsoplantar (DP), medial oblique, and lateral projection when available. Equivalent standard of care imaging (e.g., computed tomography) could be used in place of x-rays. Radiographic assessment of bone healing status was determined by the surgeon using standard of care procedures. Risk factors for nonunion were also assessed and included high body mass index (BMI), age (>65 years), diabetes, nicotine, and osteoporosis.
Pulsed Electromagnetic Field Stimulation Procedures
Following a diagnosis of nonunion, therapy with PEMF was initiated using the Orthofix PhysioStim device. The PhysioStim device is an FDA-approved non-invasive PEMF bone growth stimulator indicated for non-surgical, prescription treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than 50% of the width of the bone to be treated. A nonunion was considered to be established when the fracture site showed no visibly progressive signs of healing.
The PhysioStim device contains a control unit and a treatment coil in one integrated device. A micro-processor generates the PhysioStim device’s electrical signal, which creates a highly uniform, low-energy electromagnetic field sent from the treatment coil. When the coil is centered over the treatment area, the therapeutic PhysioStim PEMF signal can be delivered through clothing, bracing, or over a cast or external fixation device if present directly to the fracture site. Proper treatment does not require direct contact with the body however the coils must be centered around the fracture site to be effective. The PhysioStim device provides 360 degrees of PEMF treatment coverage around the targeted fracture site and penetrates evenly across tissue, bone, and fixation. PhysioStim devices provide daily treatments for up to 365 days. The physician determined the overall length of treatment (months/weeks) on an individual basis according to fracture healing progress. Typical prescribed treatment time is three hours per day as prescribed by a physician.
Statistical Analysis
Data were analyzed with SAS Version 9.4 (SAS Institute, Cary, NC). All available data collected for each subject through the study exit was evaluated for each endpoint. Subject data collected from visits were matched to the closest 1-month post-treatment interval. One visit was matched to one timepoint. Data analysis consisted primarily of summary statistics and inferential statistics for changes in dependent measures over time. The mean standard deviation (SD) is reported for continuous variables.
RESULTS
Subjects Characteristics
Fifty-six (n=56) subjects were included in the analysis. The majority of subjects enrolled were female (n=50; 89.3%), with a mean age of 59.7 years (SD 14.8) and mean BMI of 27.2 kg /m2 (SD 7.3). The types of fractures included avulsion (Zone 1) (n=21/55; 38.2%), Jones (Zone 2) (n=23/55; 41.8%), dancers/shaft (Zone 3) (n=7/55;12.7%), and head/neck (n=3/55; 5.5%). The cause of injury included direct trauma (n=19/56; 33.9%) and indirect trauma (n=37/56; 66.1%). Approximately 82.1% (n=46/56) of fractures were acute compared to 17.9% (n=10/56) of stress fractures. (Table 1) The mean time between the original fracture and the diagnosis of nonunion was 106.2 days (SD 57.3). Of the risk factors studied (BMI, age, diabetes, nicotine, osteoporosis), 75.0% (n=42/56) of subjects had at least one risk factor associated with compromised bone healing; 33.9% (n=19/56) had ≥ two risk factors for nonunion. (Table 2)
Healing Status
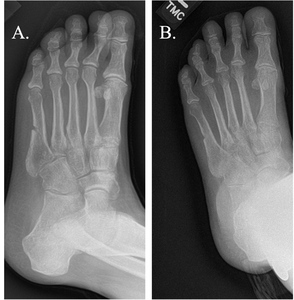
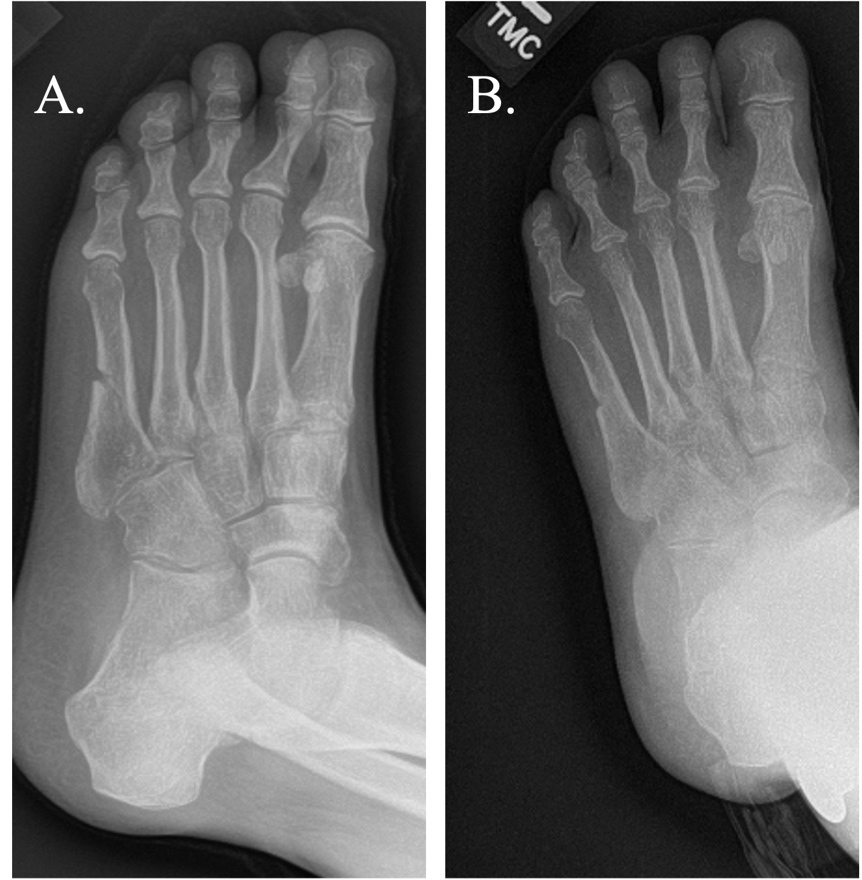
The average time from nonunion diagnosis until successful healing was 154.7 days following PEMF treatment. The majority of subjects (n=35/56, 62.5%) were categorized as healed with by four months post-PEMF initiation and 75.0% of subjects (n=42/56) were healed by six months. (Table 3; Figure 1; Figure 2) Representative radiographic images are presented in Figure 3. The number of risk factors (up to 5 total per subject) were significantly associated with longer heal times (p < 0.02). Of the individual risk factors studied, only nicotine use was associated with longer healing times (p < 0.03). (Table 4)
DISCUSSION
The current study aimed to assess the real-world impact of PEMF treatment on metatarsal fractures in patients who did not receive surgical intervention and presented with risk factors for nonunion. In total, 56 subjects were assessed, and a positive benefit was found with 91.1% of fractures healed within one year with an average time from nonunion diagnosis until successful healing of 154.7 days. The majority of subjects presented with at least one risk factor for nonunion demonstrating successful healing despite contributing factors compromising recovery. These data add to the growing literature demonstrating the beneficial effects of PEMF in musculoskeletal indications and support further investigation into the utility for metatarsal fractures.
For some patients, interruptions in the processes involved in bone repair occur leading to the development of delayed union and nonunion fractures. PEMF devices are gaining increased interest and use by patients as a non-invasive tool to mitigate impaired fracture healing. Accumulating scientific evidence supports the use of PEMF, and more specifically the musculoskeletal field is seeing a surge in research initiatives. Research largely supports the beneficial effects of PEMF in these indications and its correlation with accelerated healing of nonunion fractures. Studies have evaluated the effects of PEMF treatment for nonunion in non-surgical populations, and also as an adjunct treatment following spinal surgery with high rates of successful fusion and improved patient-reported outcomes.24,25
A recent survival analysis conducted in 1,382 patients with delayed union or nonunion fractures treated with bone growth stimulators using PEMF concluded that the median heal time for fractures was reduced by 35%-60%. The range of effect was dependent on the different fracture characteristics of patients and compliance with the recommended daily use.26 The overall success rate for fracture union was reported at 89.6% following use of PEMF devices. Increased daily compliance and hourly use of PEMF was correlated with reductions in time to heal.26 Similarly, other reports are establishing optimized PEMF treatment protocols and report higher success rates in patients who use PEMF devices regularly for longer periods of time. A study conducted in 139 subjects with nonunion fractures showed an 80% success rate following PEMF treatment in subjects that used the device > 3 hours per day (compared to 35.7% success with <3 hours use). Similar to the current study, successful healing rates were not affected by common risk factors for nonunion (e.g., duration of nonunion, age, infection) suggesting the potential for PEMF to overcome additional disease burden.27
A recent retrospective analysis of real-world data from a large claims-based database evaluated 11,010 adult patients with a diagnosis of nonunion from a prior fracture. Patients who received treatment with a non-spinal electrical osteogenesis simulator device were compared to a control group who did not. Patients who received stimulation treatments showed a significant reduction in rate of surgical intervention for their bone nonunion, lower rates of opioid utilization, and lower healthcare costs compared to those who did not receive treatment. The results indicate a positive impact of bone growth stimulator devices on individual and societal outcomes in patients with fracture nonunions.28
While studies in musculoskeletal indications are on the rise, few studies have assessed the effect of PEMF in metatarsal nonunion. Holmes et al. conducted a pilot study to evaluate PEMF effect on nine subjects with delayed union and nonunion of the proximal fifth metatarsal. Following PEMF treatment, all fractures healed with a mean time to union of 4 months (range 2-8 months). Fractures healed with a combination of PEMF as an adjunct treatment with a cast healed with a mean time of 3 months. Follow-up extending to 60 months demonstrated no refractures.29 Additionally, a prospective, randomized, double-blind trial of subjects diagnosed with fifth metatarsal delayed or nonunion were evaluated following open reduction and internal fixation of the nonunion site with adjunct PEMF or placebo stimulation treatment. A biopsy taken at study initiation of the fracture site was also analyzed for PEMF effect on growth factors. Subjects underwent repeat biopsy and the surgical procedure after 3-weeks PEMF treatment. Treatment with PEMF showed an improved time to complete radiographic union by almost 6 weeks compared to the control group indicating improved healing time following surgery. Biopsy results indicated significant increases in placental growth factor levels following PEMF with a trend for increases in brain-derived neurotrophic factor and bone morphogenetic protein (BMP)-7 and BMP-5.30 The current study supports these published reports detailing improved metatarsal fracture healing following PEMF and provides additional evidence for its utility in the large patient population suffering from metatarsal fractures. Due to the frequent occurrence of fifth metatarsal fractures, these positive findings further support investigation of PEMF as a therapeutic modality.
CONCLUSIONS
PEMF treatment is a beneficial non-invasive therapy that can be implemented as a therapy for nonunion healing. Metatarsal nonunion subjects treated with PEMF achieved favorable time to healing despite having risk factors for compromised healing. The results suggest that PEMF may attenuate compromised healing associated with known risk factors for nonunion.
Acknowledgements
We thank Stephanie E. Tedford, PhD, of Pharmacologics, Inc, who, on the behalf of Orthofix, Inc developed the first draft and assisted in implementing author revisions and Deanna Schreiber-Gregory for biostatistical analysis.
Corresponding Author
William R. Adams, II, D.P.M., F.A.C.F.A.S.
The Orthopaedic Institute of Western Kentucky
Paducah, KY, USA
wa1984@ymail.com
Data availability statement
Data supporting the findings of this study are available upon reasonable request.
Funding statement
Orthofix US LLC.
Conflict of interest disclosures
None.
Ethics approval statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Sterling Institutional Review Board (IRB) and local IRBs where applicable.
Patient consent statement
This study received a waiver of consent from the central IRB (Sterling) due to the inclusion of retrospective deidentified information.
Permission to reproduce material from other sources
Not Applicable
Clinical trial registration
Not applicable