Introduction
Osteoarthritis (OA) is a painful and debilitating joint disease that is characterized by the degenerative breakdown of articular cartilage and subchondral bone. The prevalence of hip OA is increasing, particularly among young adults, for whom hip replacement is often not well-suited to the needs of young, active patients.1 This has driven and popularized advances in regenerative medicine strategies, particularly intra-articular orthobiologic injections, which are increasingly being used as treatment alternatives for painful, OA hip joints.2 These injections may help modulate OA symptoms, improve pain and function, as well as potentially slow the degenerative breakdown of joint tissues.3,4
Currently, there are various orthobiologic injections available for treating hip OA. In the United States, hyaluronic acid (HA), platelet-rich plasma (PRP), microfragmented adipose tissue (MFAT), bone marrow aspirate (BMA) along with its concentration (BMAC), are FDA-compliant orthobiologic therapies routinely being used to treat hip OA in clinical practice, while isolated/culture-expanded mesenchymal stromal cells (MSCs) from various tissues have been used in clinical trials. HA is a naturally occurring component of synovial fluid that has been widely used in the treatment of OA but its use in hip OA has been controversial.5–8 While PRP, which delivers a high concentration of growth factors derived from a patient’s blood, has demonstrated sustained symptom improvement beyond six months post-injection.6,7,9 MFAT is a relatively new technique that harnesses the regenerative properties of adipose tissue by mechanically microfragmenting it into smaller clusters while preserving the stromal vascular fraction, which contains reparative cells and growth factors. MSCs, derived from sources such as bone marrow or adipose tissue, have been shown to be effective in address OA symptoms and improving patient reported function.10 BMA and BMAC, which contain a mix of bone marrow-derived cells and proteins, has also shown promise, with early studies indicating safety and efficacy.11 Within the literature, these orthobiologic therapies have shown to improve OA symptoms and function within patients.
Therefore, the purpose of this systematic review is to review the outcomes of these orthobiologic intra-articular injections for hip OA. Since there is large variability among clinical outcomes and follow-up time points used for these studies, we focused on the three most utilized patient reported outcomes measures (PROMs): Visual Analog Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and the Harris Hip Score (HHS), using the latest follow-up time point.
Methods
Eligibility Criteria
A comprehensive search of several databases from 2000 to July 3rd, 2024, English language, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for orthobiologic therapies for hip cartilage defects in humans. The actual strategy listing all search terms used and how they are combined is available in the appendix.
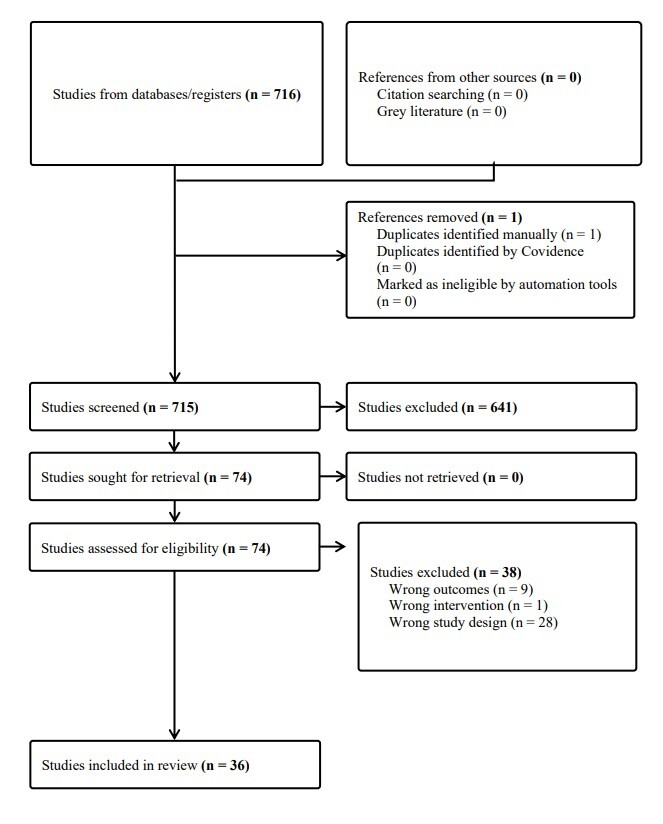
Seven-hundred and sixteen articles were identified and screened independently by two authors (C.H, P.S.). Studies eliminated by both reviewers were removed. Disagreements were resolved between the reviewers and the senior author (M.H.). Articles excluded during screening included those regarding corticosteroids, rheumatoid arthritis, surgical procedure, not published full-text, systematic reviews, or review articles. Remaining studies were reviewed for the following inclusion criteria: (1) interventions including orthobiologic intra-articular injections such as HA, PRP, MSCs or BMAC, (2) hip joint based interventions and outcomes, (3) clinical population, and (4) one or more clinical outcomes (strength, range of motion and patient reported outcome measures). Exclusion criteria was not meeting any of the inclusion criteria and studies presented as abstract at conferences without full text available.
Quality Assessment
A pair of reviewers worked independently to determine the reported methodological quality of eligible randomized controlled trials (RCT) and cohort studies. For the RCTs, the authors assessed allocation concealment, blinding of patients, blinding of health care providers, and outcome assessors. In coding the methodological quality using the Newcastle-Ottawa criteria for cohort studies, the authors assessed the adequacy of the representativeness of exposed cohort and non-exposed cohort, ascertainment of exposure, comparability of cohorts, bias protection in the outcome assessment, and adequacy of outcome follow-up. The reviewers came to a consensus on bias assessment with a supervisor.
Data Extraction and Analysis
Study characteristics, patient demographics, injection type and frequency, and outcomes including PROMs at baseline and final follow-up were extracted from each study. Due to the large amount of cohort studies, heterogeneity of variables studied and reported, meta-analysis could not be performed. A descriptive review was conducted on all the studies, and outcomes reported by each study are detailed in tables 1-4. Due to the variety and abundance of PROMs used between the studies, we investigated the top 3 most utilized outcome scores (VAS, WOMAC, HHS) at latest follow-up. These scores cover a range of clinical outcomes for pain, function and overall health post- injection.
Results
In total, 36 studies were included in this review. Studies examining multiple treatments added to the group size but did not contribute to the overall study count. There were 21 studies examining HA, 11 on PRP, 3 for MFAT, 2 for ACT/MSC, and 3 on BMA/BMAC. In total, 8 studies were randomized controlled trials, and 28 were cohort studies. HA and PRP injections were investigated in the randomized-controlled trials and cohort studies, whereas MSCs and BMAC injections were only described in cohort studies and case series.
Hyaluronic Acid
Of the 21 studies describing outcomes of HA for osteoarthritis, there were 15 observational cohort studies, and 6 randomized controlled trials (Table 1). In total, the mean patient age ranged from 45 ± 16.9 to 73.62 ± 7.87 years.
The ratio of men to women was 7:8 for the combined studies. The Kellgren-Lawrence (KL) grade had a range of 1-4, with the most prevalent grades being moderate (2-3) among the studies. The studies reported a follow-up as early as 1 week and as long as 7 years, but the latest reported follow-up was used when collecting outcome scores.
The Visual Analog Scale (VAS) for pain was most used among all HA outcome reporting scores, with 15 studies. WOMAC, HHS, and Lequesene Index followed close behind with 10, 8 and 8 studies, respectively. Non-steroidal anti-inflammatory drug (NSAID) use was reported in a total of 5 studies, and the other 7 outcome measures each were described in less than 3 studies.
VAS
15 studies reported values at baseline and at latest follow-up (3-84 months). All studies demonstrated improvement in pain following HA injection.
Nine studies used a 10 point scale to measure VAS outcomes. Nouri et al.31 showed a 4.2 point decrease (8.1±1.2 to 3.9 ± 1.4) in hip pain 6 months post-injection. Abate et al.29 (2014) reported a 3 point reduction (6.7 ± 1.3 to 1.7 ± 1.8) in pain after 12 months. Migliore et al.23 (2017) showed a ≥ 2 point reduction in pain in their cohort aged between 70 and 80 years, (7.0 ± 2.2 to 4.8 ± 1.7) at 6 months and an even further decline to 3.54±1.66 at 84 months.
DiSante et al.24 reported a 2.7 point decrease (6.3 ± 1.7 to 3.6 ± 2.1) in pain after 16 weeks. Migliore et al.14 (2020), Doria et al.,15 and Schiavi et al.19 all showed < 2 reduction in VAS score at 12 months: (6.4 ± 2.2 to 5.3 ± 1.9), (7.8 ± 1.9 to 6.1 ± 2.3), (7.2 ± 2.1 to 4.8 ± 1.8). Abate et al.18 (2017) saw a 1.2 point decrease in pain (4.4 ± 1.5 to 3.2 ± 1.2) at 6 months. Micu et al.27 saw the greatest reduction in VAS score at a 6 point decrease (8 (range 7-8) to 2 (range 2-3)) at 6 months.
Four studies used a 100 point scale to measure VAS, all of which demonstrated improvements in mean scores.^21, 22,^ 23,17 Three studies reported postoperative reduction in pain of ≥ 40 points at latest follow-up.13,16,28 Papalia et al.16 showed a 44.7 point reduction (67.5 ± 10.8 to 22.8 ± 24.1) in VAS score at 6 months. Ometti et al.28 saw a 41.1 point decrease (62.1 ± 15.2 to 21.0 ± 14.5) at 12 months. De Lucia et al.13 saw a 41.2 point decrease (range 31.1- 51.3) in a cohort of patients with grade 2 OA at 24 month follow- up. With a 3 month follow-up, Koyano et al.17 showed a 15.2 point decrease (49 ± 12 to 33.8 ± 15.2) in VAS score.
Rivera et al.26 and Dallari et al.32 reported using VAS as a tool for pain measurement, but did not report values at follow up. However, they found that they were significant different (p<0.001) after 3 and 6 months.
WOMAC
Ten studies reported using WOMAC to evaluate clinical symptoms including pain, stiffness and function following HA injection. Studies recorded values at baseline and at latest follow-up (3- 24 months). Notably, 9 out of 10 studies demonstrated improvement in mean scores following HA injection.
Six studies reported score reduction ≥ 10 points at 6 months. Doria et al.15 showed a 15 point reduction (24 ± 1.9 to 9 ± 5.6) in the pain subscale at 12 months. Nouri et al.31 and Pogliacomi et al.22 reported a 14.2 point decrease (41.4 ± 11.5 to 27.2± 9.3) and 11.9 point decrease (62.2 ± 17.0 to 50.3 ± 17.6) in total score at 6 months, respectively. Notably, Micu et al.27 reported a 50 point total decrease (57, range 45.5-64.5, to 7, range 4.8-12) after 6 months. DeLucia et al.13 reported a 34.7 (24.7- 44.7) point decrease in WOMAC total score after 24 months. DiSante et al.24 saw a 22.5 point decrease (42.36 ± 20.5 to 19.9 ± 11.4) in WOMAC pain subscale after 16 weeks.
Eymard et al.21 saw an 8.5 point decrease in the pain subscale (26.0, range 7.0-42.0, to 16.5, range 0.0–46.0) after 3 months. Brander et al.25 found a 2.21 (1.78- 2.65) point decrease in pain at 26 weeks. Dallari et al.32 used WOMAC pain as an outcome measure, did not report values, only reporting that there was significant improvement (p<0.001) at 6 month follow-up.
While most studies showed relative improvement at latest follow-up, Kraeutler et al.30 reported a 4.2 point increase (77.9 ± 14.4 to 82.1 ± 15.1) in the WOMAC total score after 6 months.
HHS
In total, eight studies used HHS, and reported values at baseline and latest follow-up (1, 6, or 12 months). All studies demonstrated an improvement in mean values following HA injection.
Five studies showed improvements ≥ 10 points at latest follow-up. Ometti et al.,28 Pogliacomi et al.,22 and Schiavi et al.19 reported a score increase by 16.5 points (71.2 ± 9.67 to 87.7 ± 9.35),15.14 points (57.36 ± 14.76 to 72.5 ± 14.71) and 14.8 points (56.4 ± 11.9 to 78.9 ± 6.1) at 12 months, respectively. Rivera et al.26 and Doria et al.15 saw a similar increase of 13.41 points (68.35 to 81.76) and 13 points (62 ± 9.8 to 75 ± 11.4) at 12 months. Long et al.12 reported a 12.83 point increase (58.47 ± 12.82 to 71.3 ± 16.46) at 6 weeks. Two studies by Abate et al.18,29 showed an improvement <10 points at 6 months (65 ± 14 to 72.5 ± 12.1) and 12 months (83.3 ± 6 to 88.2 ± 4.7).
Platelet-Rich Plasma
Out of the 11 studies examining PRP, 7 studies were randomized controlled trials, comparing PRP with HA or to a control (Table 2). The remaining 4 were observational cohort studies measuring the effectiveness of PRP to address clinical symptoms. The mean age of patients receiving a PRP injection had a range of 45.6 ± 13.1 - 71.37 ± 6.03 years. The ratio of males to females in the studies were comparable. However, Mazzotta et al.33 and Singh et al.34 had a larger number of males to females enrolled in their studies. Latest follow-up was reported, ranging from 16 weeks to 12 months. The mean pre-operative KL grade was reported in in 9 studies, ranging between grades 1-4 with the most encountered being moderate (2-3). Doria et al.15 reported the lowest mean baseline KL grade at 1.5 ± 0.5. Three studies used a Tonnis grade to determine the level of OA, which ranged from grades 1-3.
VAS
In total, ten studies reported using VAS as a tool to measure patient pain postoperatively and reported values at baseline and latest follow-up (6 and 12 months). All studies saw improvements in mean scores following PRP injection.
Six studies demonstrated a decrease in VAS pain by < 5 points. Nouri et al.,31 Singh et al.,34 and Topaloglu et al.38 reported a reduction in VAS scores at 6 months of 4.51 points (7.63 ± 1.31 to 3.12 ± 1.29), 2.6 points (6.9 ± 0.7 to 4.3 ± 1.8) and 2.15 points (7.61 ± 1.72 to 5.46 ± 2.18),respectively. Fiz et al.36 saw a decrease of 0.9 points (4.5 ± 1.4 to 3.6 ± 1.8) at 6 months.
Ortiz-Declet et al.35 found a 1.7 point reduction (4.1 ± 1.9 to 2.4 ± 2.6) and Doria et al. reported a reduction of 1.1 points (7.5 ± 2.1 to 6.4 ±2.9) at 12 months. DiSante et al.24 saw a 0.72 point decrease (7.08 ± 2.0 to 6.36 ± 2.1) after 12 months. Dallari et al.32 found a significant decrease in pain in after 12 months in the PRP group.
Two studies used the 100 point scale for reporting VAS outcome. Mazzotta et al.33 saw improvements in both comparison PRP groups, with autologous PRP seeing a 2.6 point reduction (31.8 ± 19.9 to 29.2 ± 21.0) and umbilical cord PRP seeing a 7.77 point decrease (37.17 ± 22 to 29.4 ± 24.6) in 12 months. Palco et al.37 reported a 23.07 point reduction (72.69 ± 13.65 to 49.62 ± 14.53) at 12 months.
WOMAC
In total, eight studies reported using WOMAC to evaluate total pain, stiffness and function after PRP injection. Studies reported values at baseline and at latest follow-up (3, 4, 6 or 12 months), with 6 of the 8 studies showing improvement in mean values.
DiSante et al.24 found a 5.4 point reduction (58.89 ± 22.0 to 53.47) in WOMAC pain subscale after 16 weeks. Topaloglu et al.38 reported a reduction of 10.01 points (58.16 ± 18.54 to 48.15 ± 21.45) in their treatment group, and 23.76 (55.12 ± 19.19 to 31.36 ± 22.91) in their placebo group at 6 months. Nouri et al.31 reported a 19.85 point decrease in total score (41.38 ± 9.36 to 21.53 ± 10.4) after 6 months. Dallari et al.32 did not show mean values at follow-up but reported significant improvement in WOMAC pain scores after 6 months.
Mazzotta et al.33 showed a 3.8 point reduction (27.1 ± 17.6 to 23.3 ± 18.2) in the autologous PRP group, and a 5.4 point decrease (27.9 ± 17.2 to 22.5 ± 18.3) in the umbilical cord PRP group at 12 months. Doria et al.15 showed a 16.3 point reduction (23.7 ± 2.1 to 7.4 ± 2.5) in pain subscale at 12 months.
Two studies did not show improvement following PRP injection. Kraeutler et al. saw a 9.4 point increase (67.6 ± 19.7 to 77 ± 15.8) in WOMAC total at 6 months. Fiz et al.36 showed an increase in WOMAC pain of 9.1 points (64.4 ± 16.4 to 73.5 ± 17.2) at 12 months.
HHS
In total, 5 studies used the Harris Hip Score (HHS) to report outcomes. Of those, 4 studies reported mean and SD, at baseline and at 12 months, with variable results. Mazzotta et al.33 compared umbilical cord PRP versus autologous PRP. The umbilical cord group improved by 3.4 points (78.6 ± 11.4 to 82.0 ± 14.2), while the autologous group reported a slight decrease of 0.4 points (83.8 ± 11 to 83.4 ± 14) at 12 months. Ortiz-Declet et al.35 and Doria et al.15 showed an increase of 18.6 points (70.6 ± 12.4 to 89.2 ± 10.6) and 14 points (64 ± 10.3 to 78 ± 11.3) at 12 months. Palco et al.37 reported a decrease of 13.78 points (66.86 ± 9.96 to 53.08± 24.31) at 12 months. Dallari et al.32 used HHS as an outcome measure but did not report mean values at baseline and follow-up.
Microfragmented Adipose Tissue
Of the three studies included in this sub-analysis, there was a prospective cohort, observational study, and a case series study (Table 3). Natali et al.39 was a prospective cohort study of 55 patients (22 males, 33 females; mean age 60.6 ± 16.4 years) which observed the outcomes of MFAT injections for hip osteoarthritis. Heidari et al.40 was an observational, intention-to-treat study of 147 patients (74 males, 73 females; mean age 52.5 ± 10.9 years) that compared outcomes of patients who received a MFAT or MFAT and PRP for their hip OA. There was one case series, conducted by Dall’Oca et al.,41 with a total of 6 patients (5 males, 1 female) receiving an MFAT injection. The KL Scale was used in these 2 studies to report baseline values of OA, which had a severity range of 1-4 with moderate being the most prevalent. Dall’Oca et al.41 used the Tonnis OA scale, with a range of 0-2 at baseline. Follow-up for the studies ranged from 6-35 months post- injection.
VAS
All 3 studies used VAS as an outcome measure and demonstrated an improvement in pain following MFAT injections. These studies reported VAS at baseline and at 6 months posttreatment.
Dall’Oca et al.41 reported a 3.1 decrease (4.6 ± 0.8 to 1.5 ± 0.5) after 6 months. Heidari et al.40 recorded VAS on a 100 point scale and showed a score decrease 13 points (41 to 28) at 6 months. Natali et al.39 saw a 2.9 point reduction (4.7 ± 1.1 to 1.8 ± 0.4) during their follow up between 29 and 41 months.
HHS
Only one study used HHS as a tool to measure hip function post injection which reported mean and SD values at baseline and at 6 months. Dall’Oca et al.41 reported a 17.4 point increase (67.2 ± 3.4 to 84.6 ± 6.3) at 6 months.
WOMAC
Dall’Oca et al.41 was the only study to use WOMAC as a tool to measure patient reported outcomes at baseline and at latest follow-up (6 months). This study reported a 16.5 point reduction in the total score (36.3 ± 4.7 to 19.8 ± 3.4) after 6 months.
Autologous Cell Therapy
There were two studies that used autologous cells to treat patients with hip OA (Table 4). Onoi et al.42 and Mardones et al.43 each conducted a prospective cohort study. Mardones et al.43 used cultured expanded bone marrow-derived MSCs, and Onoi et al.42 used adiposederived stromal vascular fraction (SVF), which includes a variety of cells, including MSCs, blood cells, macrophages, fibroblasts, pericytes, and endothelial cells. The SVF treatment allows for a point-of-care cell therapy which doesn’t require any cell expansion. Mardones et al.43 treated hip OA patients with MSC injections in 10 patients (5 males, 5 females) in their studies, while Onoi et al.42 treated 42 patients (5 males, 37 females) with SVF injections for hip OA. The mean patient age ranged from 49.7 ± 10.3 years to 60.2 ± 9.4. OA severity ranged from moderate to severe, and follow-up for the studies ranged from 6- 40 months post- injection.
VAS
Both studies used VAS as an outcome measure and demonstrated an improvement in pain following autologous cell injection for hip OA. Onoi et al.42 showed a score decrease of 29 points (75.5 ± 15.8 to 46.5 ± 27.9) at 6 months. Mardones et al.43 recorded a 3.1 point reduction (4.2 ± 0.5 to ± 1.1 ± 0.3) post injection with a latest follow-up between 16-40 months.
HHS
Both studies used HHS as a tool to measure hip function post injection. Onoi et al.42 showed a 20.6-point increase (25.2 ± 16.6 to 46.8 ± 27.2) at 6-month follow-up. Mardones et al.43 reported a 23.8-point increase (61.9 ± 6.1 to 85.7 ± 3.9) post- injection (16- 40 mos.).
WOMAC
Mardones et al.43 is the only study that used the WOMAC tool to measures patient outcomes.43 This study showed a 15.3 point decrease (34.5± 8.2 to 19.2± 6.1) post- injection (16- 40 mos.).43
Bone-Marrow Aspirate and BMA Concentration
Out of the 3 studies investigating BMA and BMAC hip injection efficacy, there was one cohort study and two case series.
A retrospective cohort study by Tsitsilianos et al.44 examined 31 patients, 15 male and 16 female, with an average age of 62.4 ± 16.5 years, as well as a baseline KL grade of 2.9 ± 0.7. Patients were evaluated at baseline, and at latest follow-up (12 weeks, 6 months, and 12 months). A case series by Darrow et al.45 followed 4 patients: 3 male and 1 female, with a mean age of 67 ± 10 years. The patients had OA ranging from mild to severe. All patients had a mean follow-up of 22.75 days after the last injection. Finally, a case study by Whitney et al.46 examined 16 patients, 7 male and 9 female, with a mean age of 57.6 ± 11 years. The patients had a baseline tonnis grade ranging from 2-3. Patients were evaluated at baseline, 6 weeks, 3 months, and 6 months following the injection.
WOMAC
Only one study used WOMAC pain for outcome reporting, which had a favorable result for BMAC. Whitney et al.46 showed a 6-point decrease (31± 19 to 25± 13) in pain at 1 month and a 9-point reduction (31± 19 to 22± 14) in 3 months. Finally, at 6 months patients reported a 15-point reduction in WOMAC total score (31± 19 to 16± 14).46
HHS
Additionally, one study used modified- HHS as a tool to measure outcomes post- injection. Whitney et al.46 reported a 10-point increase (63 ± 19 to 73 ± 15) in hip function at 1 month post-injection and a 16 point increase (63± 19 to 79± 15) 3 months from injection. Finally, at 6-month follow-up, patients reported a 17-point increase in mHHS (63± 19 to 80± 15).46
Discussion
The goal of this systematic review is to provide a comprehensive evaluation of clinical outcomes following intra-articular injections for hip OA. We focused on the most commonly used biologics, including HA, PRP, MFAT, BMA/BMAC, and MSCs, all of which have been more extensively studied in knee OA and are now being increasingly applied to the treatment of hip OA. Among the biologics used, HA and PRP were the most extensively studied, while BMAC, MFAT and MSCs report promising findings, the literature for these “cell-based therapies” in hip OA is scant. Because of the heterogeneity among the studies, our review focused on the subjective outcomes of VAS, WOMAC, and HHS at the studies respective final follow-up time points. These measures were the most frequently utilized to measure pain and function for patients at baseline and follow-up.
Our findings indicate that intra-articular orthobiologic therapies for hip OA are associated with improvements in PROMs. Zaffagnini et al.47 also performed a systematic review of orthobiologic injections for the treatment of hip OA to evaluate the safety and efficacy of various products. These authors found that there was an overall improvement in pain and function in hip OA patients treated with orthobiologic injections and that most improvements were observed within the first 6 months.47 While we did not evaluate safety in our study, we found similar results in that orthobiologics used to treat patients with hip OA also had improvements in pain and function at the studies final follow-up time points.47 Acuna and colleagues48 performed a systematic review on viscosupplementation for hip OA and focused on whether PROMS support its use. We similarly found that VAS and WOMAC outcomes focused on pain and function improved following HA administration for hip OA.48 Interestingly, this group also found that formulation of HA did not appear to influence outcomes, and that total hip arthroplasty conversion was low, but this may be due to the range of follow-up being between 1- and 4-years of follow-up.48 Furthermore, Xiong et al.49 performed a systematic review and meta-analysis of randomized controlled trials that used PRP for the treatment of OA for all joints including the hip. Notably, this study found that PRP was safe and effective for knee and ankle OA, but the results for hip OA did not demonstrate a significant reduction in pain among patients.49 This may be attributed to the only 2 studies that were included that used PRP for hip OA and the limited sample size.49 Overall, our findings support the role for intra-articular injections of orthobiologics to alleviate hip OA symptoms.
Importantly, this review found significant heterogeneity among the studies, including using different orthobiologics with specific methodological preparations, studying a wide range of sample sizes, and having various follow-up time periods, all of which limit our ability to make direct comparisons between the studies. Of note, there were a few studies that had a long-term follow-up period, including Mazzotta and colleagues33 who followed their patient cohort for 7 years. Additionally, each study in this systematic review followed patient reported outcomes post-treatment for various periods of time, not allowing for direct comparisons. While the PROMs have demonstrated efficacy of orthobiologic injections to improve pain and function, there is not enough evidence of sustained benefits beyond 1 year. This was also noted by Zaffagnini et al.47 who reported in their systematic review that some authors reported a gradual worsening of clinical outcomes toward the end of their study follow-up.
This review highlights several limitations in the current literature. A vast majority of the studies included were single-arm, pre-post studies with unblinded evaluations. As such, causal inferences about the effectiveness of the interventions cannot be made. Second, there was variability in duration of follow-up for the studies, ranging from 1 week to 84 months. There was also a lack of consistency regarding the types of PROMs used, and how the values were reported, making it difficult to effectively compare scores at baseline and follow-up. Also, there was a wide range of sample sizes between the studies, creating a barrier to draw conclusions about the efficacy and long-term effects of orthobiologic injections of the hip.
Although the current literature has several limitations, a strength of this study is the comprehensive and systematic review approach. We also assessed the methodological quality of the included randomized controlled trials and observational cohort studies, accordingly.
Although there have been previous reviews of the literature, to our knowledge, this is the first systematic review investigating outcomes of all biologic injections for the hip that are used in practice.
Conclusion
This systematic review demonstrates promising results for orthobiologic injections including HA, PRP, BMAC, MFAT and MSC injections for the treatment of hip OA. While we found a broad range of PROMs used among our included studies, focusing on the three most utilized measures (VAS, WOMAC, and HHS) allowed us to evaluate the relative impact of these intra-articular injections on pain and function in patients with hip OA. Future research should focus on conducting rigorous randomized controlled trials and assessing outcomes beyond 1 year.
Corresponding Author
Mario Hevesi, MD, PhD
Assistant Professor
Department of Orthopedic Surgery
Mayo Clinic
200 First Street SW Rochester, MN, 55905
Authors Contributions
Scott: Ms. Scott led the study and was involved with screening of articles, data synthesis, and manuscript preparation and review.
Hegarty: Along with Ms. Scott, Mr. Hegarty helped lead the study with screening of articles, data synthesis, and manuscript preparation and review.
Kang: Mr. Kang helped with manuscript preparation, data review, and clinical insights.
Boettcher: Dr. Boettcher was involved with clinical understanding of data, review of the manuscript, manuscript preparation, and important clinical insights.
Sellon: Dr. Sellon helped with clinical insights, review of the data, manuscript preparation and review.
Krych: Dr. Krych helped with manuscript preparation, review, and clinical insights.
Nagelli: Dr. Nagelli helped with developing the study, refining the research questions, involved with data review, manuscript preparation and review.
Hevesi: Dr. Hevesi was involved with formulating the idea for the paper, data review, manuscript preparation and review, and clinical insights.
Conflicts of Interest
There are no conflicts of interest.