Introduction
In sporting injuries, the superficial medial collateral ligament (sMCL) is the most commonly damaged knee stabilizer.1–7 Since it has great healing potential,4,5,8 most clinicians recommend conservative treatment in almost every case, not considering that it belongs to a functional unit: the medial soft-tissue complex of the knee (MSC). A small lesion in the setting of another MSC structure tear or a multi-ligament injury completely alters joint biomechanics. If left untreated, it may cause pain, instability, and overload other stabilizers.8,9 Nevertheless, few well-designed studies have been published around this topic, and most overlook the other MSC structures.2,10,11 This has led to misunderstanding and controversy around MSC injuries, with some authors considering it the neglected side of the knee.12,13 Consequently, this has generated growing interest in research groups, and new data has arisen from their publications. After conducting a comprehensive literature search using a computer-based search within online databases from 1970 to January 20th 2021 for randomized controlled trials, meta-analyses, systematic reviews, narrative reviews, expert consensus, and observational studies around the medial side soft tissue structures of the knee, we aim to present the current concepts, controversies, and future challenges on the issue.
Anatomy
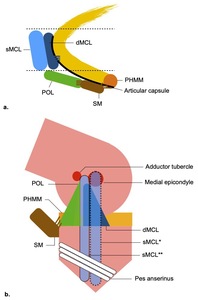
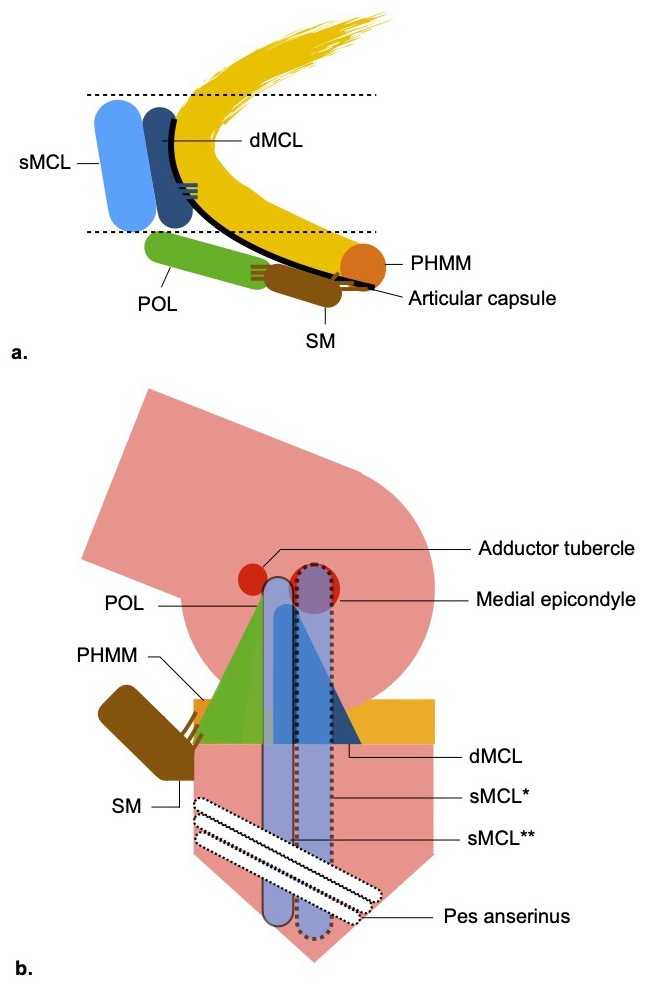
The medial side of the knee is composed of several structures that extend from the medial edge of the patellar tendon to the medial edge of the medial head of the gastrocnemius.1,2,4,5,14–16 Definitions and anatomic descriptions have changed over the last decades, leading to confusion and misunderstanding of the nomenclatures.2,4,5,17 Emerging papers have clarified this issue, and it is now accepted that the medial side structures that work as a functional unit are2,4,5,17,18: superficial medial collateral ligament (sMCL), deep medial collateral ligament (dMCL), posteromedial corner (PMC) (Figure 1). This latter is comprised of 5 individual structures that lie between the posterior border of the sMCL and the medial margin of the posterior cruciate ligament (PCL)4,19: posterior oblique ligament (POL), posterior horn of the medial meniscus (PHMM), semimembranosus distal tendon (SM), oblique-popliteal ligament (OPL), articular capsule. Topographically, in the layer by layer approach of Warren and Marshall,20 we can find the sMCL, POL, SM in layer 2, and the dMCL, articular capsule in layer 3. Going from anterior to posterior, according to Sims and Jacobs division,21 we can find in the mid-third the sMCL and dMCL, and in the posterior third the structures of the PMC. These compartmentalizations give surgeons a useful tool for a systematic approach during diagnosis and surgery.22
While some authors use a different terminology by referring to all medial side soft tissue structures as PMC,11 the majority of authors consider the sMCL and dMCL to be separate from PMC in agreement to the topography described,1,2,4,5,13,16,17,19,21 hence the designation medial soft-tissue complex (MSC) which includes the sMCL, dMCL and PMC structures.
The sMCL extends 10-12cm from proximal to distal, but there is still debate around its femoral attachment. Historically, it was reported to be attached to the medial epicondyle (ME).16,21,23 Still, more recent publications4–7,14,24 have identified a different attachment based on the findings of the seminal cadaveric study of LaPrade et al.15 These authors described the sMCL femoral insertion as being 3.2mm proximal and 4.8mm posterior to the ME. However, emerging studies published by the Imperial College London1,18 demonstrated that the sMCL covers the ME, having the attachment centered 1-2mm proximal. This controversy may be of utmost importance for surgical procedures since a non-anatomical graft positioning may alter the joint’s biomechanics. The tibial insertion is more consensual: a proximal site 10-12mm distal to the joint line (primarily to soft tissues) and a broader one 42-71mm from the joint line.15,16,18
The dMCL is an important independent stabilizer, despite being adherent to the articular capsule.1,5,15,16,23,25 It is proximal to the sMCL (6mm distal and 5mm posterior to the ME) and 15-17mm above the femoral articular cartilage.16,18 Running distally from posterior to anterior, it ends in a fan-wide tibial attachment around 8mm distal to the joint line.18 It has two major expansions to the meniscus: meniscofemoral and meniscotibial ligament.
The POL is composed of a group of obliquely oriented fibers that extend from the distal tendon of the SM towards the femur, anteriorly blending with the posterior margin of the sMCL and posteriorly reinforcing the articular capsule. Historically, it was considered an oblique part of the MCL,20,26 a reinforcement of the posteromedial articular capsule linked to the SM,16 or an individual structure.21,27 Nowadays, the latter is accepted by most literature, given its discrete location 6.4mm posterior and 7.7mm distal to the adductor tubercle.15
The distal tendon of the SM attaches to the medial and posteromedial tibia just below the joint line and creates an “octopus-like weave” with several expansions to PMC structures.15,16,20,22
Finally, the PHMM is related to the posteromedial capsule, the SM, and the dMCL.13,19,21
Biomechanics
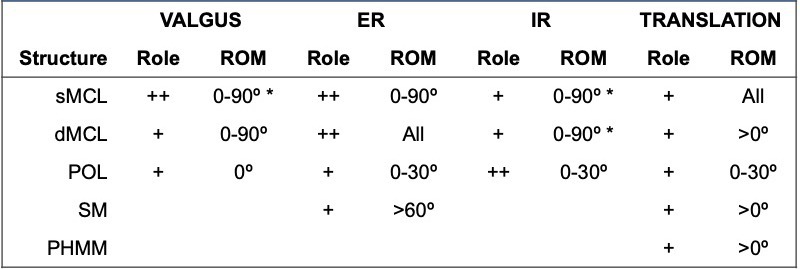
Based on the anatomy, one can easily conclude that each structure will act differently when submitted to external forces (Table 1). But studies have also demonstrated that they work as a complex unit in response to valgus and rotational forces, with the sMCL, dMCL, and POL slackening or tightening at different degrees flexion.1,9,16,28,29
Valgus forces are resisted primarily by the sMCL in all range of movement (ROM) but especially at 30º, with the dMCL acting as a secondary restraint in all ROM, and the POL as a stabilizer in extension.1,9,28–30 When these structures are injured, the anterior cruciate ligament (ACL), which has been shown to resist valgus forces, may become overloaded.1,9,10
Internal rotation forces (IR) are resisted primarily by the POL, mainly close to extension,1,9,16,28,29 although a study found it to be relevant through all ROM.28 The dMCL and sMCL also restraint IR between 0-90º, especially at 30º of flexion.9,28
When submitted to external rotational (ER), emerging evidence demonstrated that the dMCL is the major stabilizer of MSC in the extended knee. Previous studies have described the dMCL as having a secondary role resisting ER.22,28 Still, new biomechanical tests have shown that due to its oblique fibers that run from posterior to anterior, it is perfect to resist ER.1,9 The sMCL and POL also resist ER from 0-90º and 0-30º of flexion, respectively.1,9,28,30 Also, by generating IR of the tibia and tensioning structures of the PMC during flexion, the SM becomes a dynamic stabilizer against ER and anterior translation forces acting on the tibia.21,31
The MSC also has a role in controlling sagittal movements. Expansions of SM, dMCL, articular capsule, and POL stabilize the meniscus to the tibia.13,19,21,31 If these didn’t exist, during flexion, the medial meniscus and the femoral condyle would move as a unit sliding over the tibial plateau, and the so-called “brake and stop” function would be lost. Also, as the PMC tightens in extension, it contributes to control posterior tibial translation.30
Finally, one should remember that knee motion produces multi-directional forces on all its stabilizing structures, and an individual injury may alter load-sharing relationships.21,32 When all MSC stabilizers become insufficient, the so-called anteromedial rotatory instability (AMRI) may occur: abnormal medial opening and rotatory subluxation of the anteromedial tibial plateau.5,9,14,21,25,33
Diagnosis
Most literature underestimates the complexity of these injuries by characterizing them as “medial collateral ligament tears.” Even though it is true that in most cases MSC injuries are isolated sMCL tears, clinical examination and imaging should not be oversimplified, as the outcomes change depending on the knee stabilizers damaged.2,6,8,9,11,17 Proper diagnosis requires understanding if the injury is acute (<3weeks after trauma), subacute (3-6weeks), or chronic (>6weeks).34 It is also essential to understand which structures are damaged: isolated sMCL, sMCL and another MSC structure, multi-ligament injuries (involving the MSC and at least the ACL, PCL, or posterolateral corner).2,4,5,8,11,13,34
Clinical assessment
Injuries may occur after a direct impact on the lateral side of the knee or during cutting and pivoting movements. When valgus and ER forces act simultaneously, there is a higher probability of multiple structures of the MSC being damaged or multi-ligament patterns to occur (most commonly a concomitant ACL tear).3–5,7,13,17 As a result, the examination should include looking for ecchymosis, trigger points of pain, assessing the medial side’s stability, and a complete evaluation of all knee ligaments starting with the joint in a reduced position and comparing it with the contra-lateral knee.5,17 Assessment in acute cases is often complicated by pain, swelling, and effusion, but after a period of protection, pain management, and rest, the knee should be fully examined. MSC is specifically tested with three maneuvers. Firstly, a valgus load quantifying the medial opening through ROM should be applied, as it relates to the severity of tears. If there is increased laxity only at 30º, the sMCL should be the major MSC component affected, but if there is instability at 0º, an injury to the dMCL and PMC is highly likely to coexist.4,5,8,11,19 Secondly, the anteromedial drawer should be performed by applying anterior translation and an ER force at 80-90º of flexion with the foot externally rotated by 10-15º. If an anteromedial subluxation of the medial tibial plateau is noticed, the test is positive, and if associated with valgus instability, it confirms the diagnosis of AMRI. This traduces injury of the sMCL, dMCL, and POL, and possibly the ACL.2,13,14,24,31,35 Lastly, the dial test can also diagnose pathology of the MSC if there is more than 5º of asymmetry in ER. The direction of the tibial subluxation differentiates it from an injury of PLC: posterolateral for PLC, anteromedial for MSC.5,6,17
We must underline that in cases of tibiofemoral dislocations or high energy trauma need emergent care. It is mandatory to evaluate perfusion and the lower limb’s neurologic status and be vigilant for compartment syndrome.5,17
Imaging assessment
Protocols should always include standard plain radiographs to exclude fractures, bony avulsions, and dislocations.2,17,24 In chronic injuries, long leg films should be done to characterize the mechanical axis.5,11,13,17,24
Bilateral stress x-rays under valgus load are reliable in quantifying instability: 3.2mm side-to-side difference of medial gap opening represents a complete sMCL tear, and ≥9.8mm insufficiency of all major structures of the MSC.36 Although they provide important information in chronic cases, their use in the acute setting is very limited due to pain.2,5,8,24
Since multiple soft tissue structures may be affected, magnetic resonance imaging with its better anatomic definition provides essential information to evaluate the extent of injuries.2,4,5,8,11,17,37
Injury Classification
Multiple classification systems exist, but none is validated to characterize the MSC injuries.2,11 The majority of literature uses the American Medical Association modified by Hughston,17,38 which groups injuries according to the integrity of sMCL fibers and magnitude of instability under valgus load. The severity of fibers injured is graded from I to III, where grades I and II indicate sMCL pathology but no complete tear, whereas grade 3 implies a complete tear of the sMCL. The degree of instability in the latter is then grouped according to the magnitude of medial side opening in +1: ≤5mm, 2+: 5-10mm, 3+: >10mm.
Management of MSC injuries
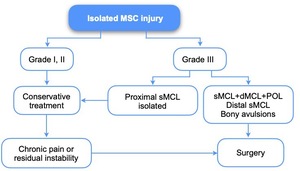
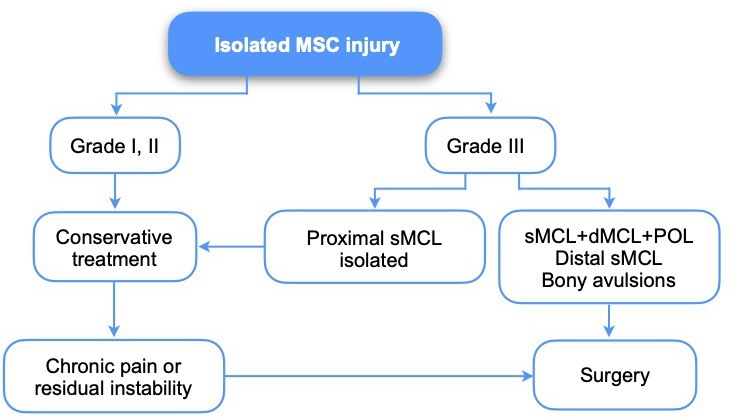
Developing an algorithm based on randomized control trials (RCTs) is complex due to the diversity of injury patterns, conservative and surgical protocols, and patient activity level and expectations. Nevertheless, we tried to summarize MSC injuries management (Figure 2, Figure 3) according to the most currently agreed.3–6,8,10,11,13,17,24,34,35,39–43
In any acute injury, the first step is to let the soft tissues rest and provisionally stabilize the knee until it is possible to make a proper diagnosis. Consequently, pain management, application of a hinged knee brace limiting ROM to 0-30º of flexion, touch toe weight-bearing (WB), and avoiding ER of the foot is recommended.5,17
MSC isolated
The sMCL is a highly vascularized structure located in the periphery of the knee, which gives it great healing potential, particularly for proximal injuries where there is a better supply.37,42 Literature has reported excellent outcomes with conservative treatment in acute proximal injuries, although there is a lack of high-level evidence defining the optimal protocol.2,4–6,8,11,13,17 Most seem to support using a ROM brace for six weeks in grade I and II injuries, with WB as tolerated,4–6,17,34,42 but in grade III, there is no consensus as to the need for a hinged brace and non-WB. For the latter, some support restricting ROM to the first degrees of flexion and non-WB for two weeks, followed by two weeks of 0-90º of flexion and WB as tolerated, then slow progression aiming to full ROM and WB at six weeks.5,17,34,42 Others are more liberal, not advocating any limitation of flexion.11
Nevertheless, there is consensus around the need to avoid ER of the foot, commence isometric quadriceps contractions, mobilize the joint within the defined ROM, followed by muscle strengthening and proprioception training after brace removal.6,11,17 Progression of rehabilitation should be made individually, guided by the onset of persisting pain and effusion and level of neuromuscular control.43 Nonetheless, grade III injuries may not heal and generate chronic pain and residual instability.1,2,4–6,44,45 Importantly, these injuries rarely occur in isolation, and in up to 80% of cases a multi-ligament pattern is found.7,8,42,46 Therefore, it is of utmost importance to evaluate other knee ligaments. On the other hand, all bony avulsions of the sMCL and distal grade III avulsions of sMCL with displacement seem to have a bad prognosis, so most recommend surgery for these cases.2,3,5,6,8,11,37
MSC in multi-ligament setting
The controversy starts from the beginning of decision making: treat MSC injuries conservatively during six weeks and reassess with the patient anesthetized at the time of the reconstruction/repair surgery of other structures, or surgical treatment of all injured structures within three weeks if soft tissues allow. As explained before, it is very complicated to develop a well-designed RCT. Most literature seems to support the idea of a more conservative approach since most sMCL injuries heal well.2,11,17,34,42 But in the context of a complex MSC injury that generates AMRI, there seems to be a higher risk of residual instability that may overload other knee stabilizers, resulting in poorer clinical outcomes and a higher risk of failure of other repairs or reconstructions.6,9,10,14,35,39,41,42 In these cases, it may be beneficial to operate as soon as possible since there is better biology and less scarring to perform repairs in the first weeks after injury.2,5,17,24,43 On the other hand, there are concerns of a higher risk of arthrofibrosis and infection with this approach, especially in high energy injuries.3,5,8,17,46 Also, some papers showed no clear benefit in clinical outcomes when comparing primary repair with conservative treatment of MCL grade III injuries in the setting of concomitant ACL injury, although these studies had important limitations.3,46 To provide some guidance, current literature seems to consider the following injuries as having poor outcomes when the MSC is treated conservatively (figure 3): combined PCL grade III or bi-cruciate and sMCL grade III,14,17,42 ACL and sMCL grade III with AMRI,1,4,6,9,10,14,17,19,24,35,41 ACL and sMCL grade III that after 3 weeks of conservative treatment are grade III 2+ or 3+ in elite athletes,5,17 tibial sMCL avulsions,2,4,5,8,13,14,17,24,34,42 sMCL entrapment,2,4,17,34,42 bony avulsions,2,4,6,8,34,42 concomitant displaced meniscus.4,5
Another controversy comes when choosing the procedure: repair, reconstruction, or repair and augmentation. Isolated repairs consist of a layer-by-layer approach, fixing damaged MSC structures with suture anchors and heavy sutures.4,5,8,17,22,34 They have the advantage of reestablishing anatomy and proprioception, but seem to be less resistant to loads than reconstruction or augmentation procedures.2,17,35,43,45,47,48 Most importantly, they may not be sufficient as an isolated procedure in subacute (3-6 weeks) or chronic (≥6 weeks) injuries because scarring results in poor tissue quality and anatomy distortion.2,5,17,43 Regarding reconstruction, literature seems to support techniques that address both valgus and rotational instability where they are present.2,11,24,29,34,41,43,49 There are several different procedures described, and they are defined as being non-anatomic or anatomic constructs.2,8,34,42,43,45,49–51 A level IV meta-analysis from Delong et al.49 analyzing the results of reconstructions in 359 patients found better outcomes for constructs defined as “anatomic,” but also a high heterogeneity of injuries, techniques, graft choices, and tensioning. Only 1 out of 25 studies (28 patients) met the inclusion criteria for “anatomic reconstruction”.50 Besides, emerging evidence1,18,29 questioned the sMCL femoral insertion and underlined the importance of the dMCL as an ER stabilizer for which there is no reconstruction technique addressing it to our knowledge. As for graft choice, there is no strong evidence guiding it.5,8,49,52 Interestingly, despite some laboratory papers showing that hamstrings contribute to valgus stabilization questioning its use as grafts in ACL reconstructions with sMCL injuries,53,54 a recent clinical study on ACL reconstruction and MCL injuries treated conservatively showed no significant differences in survivorship or worse patient-reported outcomes if hamstring autografts were used in reconstruction. Still, it didn’t specify the severity of MCL injuries.55 In terms of graft positioning and tensioning, despite sMCL fibers not being truly isometric,1,9,19,29 some authors recommend testing for isometry before fixing the sMCL graft,5,17,34,40,45,51 and tightening it at 20º-30º of flexion and slight varus force.41,50 As for the POL graft, most agree it should be fixed in full extension and neutral rotation to avoid the inability to fully extend.1,29,41,50 The third surgical option we mentioned is to repair and augment the structures of the MSC, either with soft tissue advancement procedures, synthetic tape/mesh, or reconstruction techniques. Theoretically, they would preserve anatomy and proprioception, and at the same time, provide a “seat-belt” that would deliver adequate stability for early mobilization while repaired structures heal. Still, there are concerns of overconstraining the joint.4,5,17,48,56 Two cadaveric studies have shown good results in terms of stability,47,50 but there are very limited clinical studies to prove that better outcomes are found with this approach.2,4 So, there is lack of strong evidence to determine the correct approach, but seems it seems beneficial to perform augmentation techniques over isolated repair techniques in acute (≤3 weeks) or subacute (3-6 weeks) injuries,2,6,13,17,24,35,41,43,45,47,48,55 and to reconstruct in chronic ones (≥6 weeks).2,4–6,11,24,34,42,43 For the latter, if a valgus malalignment is found, a corrective osteotomy is recommend.4,8,11,34
With regards to complications, overall, the most common one is arthrofibrosis (17-20%), followed by residual instability.4,8,24,34,47
Post-operative rehabilitation
The perfect rehabilitation program would be the one that protected structures while they are healing yet at the same time stimulated early muscle activation, joint movement, and load to speed recovery, avoid arthrofibrosis and chondrolysis. But how to balance safety and speed remains a challenge, and most studies are based on level 5 published evidence.11,50,57 Nevertheless, it is generally agreed that rehabilitation is divided into tissue protection, restoration of neuromuscular control, and optimizing function.57
In the tissue protection phase, isometric activation of the quadriceps, the use of a brace restricting flexion and hyperextension, limiting weight-bearing (WB), and rotation are recommended. The duration and level of restrictions are guided by the most fragile repaired/reconstructed structure and the one that has the greatest healing time.4,56 Most seem to recommend ROM in a safe zone determined by the surgeon for two weeks (usually <90º), followed by a progressive increase to free ROM in the following weeks, being full WB only allowed at six weeks.34,50,57 Other authors are more liberal, recommending two weeks of non-WB for the first two weeks, followed by two weeks of partial and full WB.17 Despite the controversy, isolated repairs are not as resistant as augmented repairs or reconstructions.10,35,47 Consequently, one should consider the need for higher protection and slower easing of restrictions in this phase.
After this early stage of rehabilitation, the goal is to reestablish full ROM and normal gait as soon as possible, improving strength and proprioception. Progression is staged, being the frequency and intensity of exercises implemented in an individual base. One should be aware that persistent 5/10 pain for 12 to 24 hours or effusion after exercises are signs that load must reduced.57 Also, simultaneous PCL reconstruction always changes the protocol so that excessive posterior tibial translation is avoided by using dynamic braces and restricting hamstrings strengthening for at least eight weeks.57,58
Return to sports (RTS) should be permitted only after evaluating activity-specific functional tests in a pain and effusion-free knee.50,57 The average time of RTS in isolated injuries is 6 to 9 months after surgery, but it is greater and highly variable in multi-ligament injuries, depending on the age of the patient, structures involved, the timing of surgery, type, and level of activity/sport, being up to 9-12 months with an RTS mean rate of 50%.6,57,59,60
Conclusion
The medial soft-tissue structures work as a unit to stabilize the knee from valgus, translational and rotational forces. Consequently, clinicians should understand three different injury patterns: isolated sMCL, isolated MSC, MSC in a multi-ligament setting.
Although most isolated injuries have an excellent prognosis with nonoperative treatment, in the setting of coexisting multi-ligament tears and AMRI, bony avulsions, distal sMCL, PCL grade III, or bi-cruciate injuries conservative treatment of MSC structures leads to poor outcomes, and residual insufficiency of MSC structures may influence other grafts survival. Where surgery is indicated, controversy still exists regarding the best procedure in acute and subacute tears. Still, most current literature seems to support the use of repairs and augmentation over isolated repairs. In chronic ones, reconstruction techniques that provide valgus and rotational control seem to be the most supported option. Importantly, recent biomechanical evidence has underlined the relevance of the dMCL and delivered new data on the femoral attachment of the sMCL. These findings may be a game-changer in reconstruction techniques.
The lack of high-level evidence around medial side soft tissue injuries’ clinical management still exists. Nevertheless, emerging basic science and clinical knowledge are challenging existing concepts and potentially changing future directions.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical standard
The authors declare they complied with all ethical standards.