Introduction
Spinal epidural lipomatosis (SEL) is a rare condition characterized by an overgrowth of unencapsulated adipose tissue in the extradural space.1,2 This pathologic process leads to narrowing of the spinal canal and compression of surrounding neural structures.2 Clinical presentation includes myelopathy, radiculopathy, neurogenic claudication, loss of sensation, difficulty voiding, lower extremity weakness, and rarely cauda equina syndrome.3 The most common cause of SEL is long-term exogenous steroid therapy. SEL is also associated with obesity, overproduction of endogenous steroids, surgery, or deemed idiopathic.1,4 Timely management is imperative to avoid extensive disease progression and to preserve one’s quality of life. Management of SEL is patient-specific and aimed at treating the underlying cause of disease. Conservative treatment includes reduction or discontinuation of offending steroid medications and weight loss. In those with persistent or severe symptoms, surgical decompression is indicated. The purpose of this review is to provide a comprehensive update on the epidemiology, pathogenesis, and management of SEL.
Methods
A review of the current literature was performed in PubMed, Google Scholar, Embase, and Medline from January 1, 1975, to April 31, 2021. Search terms included epidural lipomatosis, spinal epidural lipomatosis, lumbar epidural lipomatosis, spinal decompression, steroids, obesity, and minimally invasive. We sought to identify reviews, prospective, retrospective, and original studies. Analysis was performed to further clarify the epidemiology, pathophysiology, diagnostic workup, and treatment of SEL.
Clinical Presentation
Symptoms of SEL are non-specific and most commonly present as myelopathy, radiculopathy, sensory disturbances, or claudication.5,6 While some patients may have back pain, weakness, paresthesias, or ataxia early on, others may remain asymptomatic until their disease has progressed to late stages.7,8 On rare occasions, cauda equina syndrome and acute paraplegia have been the presenting signs.3,9,10 The wide range of symptoms is partly due to adipose accumulation in different regions of the spinal canal.1 Symptoms may develop acutely; although, most cases develop over months to years.11–13 Praver et al. found that higher severity of presenting symptoms was associated with a greater likelihood of delayed recovery.14
Epidemiology
The first case of SEL was reported by Lee et al. in 1975 in a patient on long-term exogenous corticosteroids following renal transplant.15 In 2015, Theyskens et al. assessed 28,902 MRI scans and found the overall prevalence of SEL to be 2.5%. Stratified further, they reported prevalence of SEL with spine-related symptoms, 1.8%, incidental SEL, 0.6%, and SEL with SEL-specific symptoms, 0.1%.6 Another study assessed 831 patients with spinal stenosis. They estimated the overall prevalence of SEL to be 6.26% and the annual incidence to be 2.5%.16 Most recently, a study performed in Korea found the prevalence of SEL to be 1.1%, with a higher prevalence in males and overweight patients.5 Before these studies, Borré et al. reviewed 2,528 MRI scans and estimated a prevalence of 21%.17 The distinction between Borré et al. and the three more recent studies was the criterion for diagnosis. Borré et al. diagnosed SEL based on a rudimentary accumulation of epidural fat in the spinal canal, which they termed grade 1. The two more recent studies only included patients with a distinct excess of epidural adipose tissue, excluding patients considered to be Borré Grade 1. Thus, the more stringent diagnostic criteria are likely more accurate and better approximates the true prevalence of SEL in the population. However, further research is needed to truly appraise the prevalence of SEL.
SEL typically occurs more commonly in males than females.5,6,16,18–20 Although rare, the condition has also been reported in children. One study assessed 125 children with renal disease, five out of 125 patients were diagnosed with SEL, and all five children were on methylprednisolone pulse therapy.21 The youngest in the cohort was five years old. Other studies have also reported on children with SEL.22–24 A unique presentation was in an 18-month child with no steroid use or endocrinopathies. The patient had an MRI performed for a delay in walking and was found to have SEL. This discovery may have been incidental; he was treated with conservative management and was still unable to ambulate at the time of publication.
Pathogenesis
The underlying pathogenesis of SEL remains largely unknown; however, irrespective of the mechanism, the condition arises due to an excessive accumulation of adipose tissue in the spinal canal.25–27 Epidural fat accumulation typically occurs slowly and will demonstrate a range of symptoms over time.28 In the initial stages, the thecal sac is diminished until it is gradually obliterated in late stages.28 Adipose deposition may cause mass effect or venous engorgement, leading to spinal cord or nerve root compression.29,30 Of note, a small mass can easily compress the thoracic cord due to its narrow width, limited vascularity, and a more significant proportion of epidural fat.18
Though SEL is exclusively localized posterior to the cord in the thoracic (T4-T8) or lumbar (L4-L5) vertebrae, the adipose tissue characteristically deposits in different locations depending on the etiology of the disease.29,31 A study by Fogel et al. reports that 55.8% of SEL cases caused by exogenous steroids affect the thoracic spine, compared to 32.7% that only involves the lumbosacral region and 11.5% that affects both. This differs from the endogenous steroid disease-related SEL, which affects the thoracic and lumbosacral areas relatively the same. In addition, the majority of obesity-related SEL (69.6%) and idiopathic-related SEL (50%) results in lumbosacral involvement.26 It is still unclear why specific etiologies have preferences for different locations of the spine.
Risk Factors
Exogenous steroids
Exogenous steroid use is considered the most common cause of SEL.1 Steroids stimulate glucocorticoid receptors in adipose tissue due to overlap between receptors.32,33 As such, chronic use of steroids hypertrophies spinal adipose tissue, leading to neural impingement and compressive spinal cord pathology.12,31 SEL has been documented in conditions commonly managed with exogenous steroids such as organ transplantation, Crohn’s disease, nephritic syndrome, prostatic cancer, pineoblastoma, lichen ruber planus, cerebral lymphoma, diabetes mellitus, multiple sclerosis, COPD, and ulcerative colitis.26,31,34–39 Several studies estimate that around 50% of SEL cases were associated with exogenous steroid use.4,26 More recently, it was reported that 27% of cases received exogenous steroids.16
Of note, one study found a positive correlation between the number of steroid injections and SEL incidence.40 However, in a subsequent study, a mean cumulative dose of steroids needed to initiate SEL could not be determined.14 This remains a topic of investigation.
Overproduction of endogenous steroids
An overproduction of endogenous steroids is also associated with the development of SEL. Cushing syndrome, carcinoid tumor, hypothyroidism, and pituitary prolactinoma have all been associated with SEL.41–47 In contrast to exogenous steroids, Fogel et al. estimated that only 3.2% of cases were related to endogenous hormonal steroid disease.26 The pathogenesis is analogous to that of exogenous steroids. Excess steroids lead to expansion of adipose tissue in the Cushingoid fat distribution and epidural space, which compresses surrounding neural structures.31
Obesity
Obesity is considered the most common cause of SEL unrelated to steroid use.1,26,48 It was found that 24.5% of SEL cases were associated with obesity-related factors.26 Obesity is thought to cause chronic inflammation and subsequent hypertrophy of adipose tissue in the spinal canal.1,49,50 The levels of inflammatory cytokines such as TNF-alpha and IL-beta were significantly elevated (roughly 2.6-fold) in obese patients with SEL.50 These cytokines likely enhance adipocyte growth, which explains the increase in adipocyte size in the epidural region compared to the non-obese control group. Borré et al. reported 80% of patients as obese, Sugaya et al. reported 60% of cases as obese, and Malone et al. reported 79% of cases as obese.16,17,51
Although obesity is a widely accepted risk factor for SEL, some studies have found no correlation between BMI and SEL. For example, in 2016, 28 patients were analyzed, 14 controls with degenerative disk disease, and 14 patients with SEL.52 The results showed no statistical significance between SEL and BMI, medical comorbidities, steroid injection, or endogenous steroid disease. In another study, there was no statistical significance between the thickness of adipose tissue in the spinal canal and BMI or waist circumference.53
Spine surgery
Spine surgery has been shown to increase adipose tissue accumulation in the epidural space. Greenish et al. reported a case of SEL acutely after spinal canal decompression surgery at L4/L5.11 The patient initially presented with claudication and bilateral leg pain, which resolved immediately after surgery and led to early discharge.11 Two days later, the patient re-presented with difficulty walking and bilateral leg pain. A clinical diagnosis of an epidural hematoma was considered; however, postoperative MRI showed an accumulation of epidural fat at L5/S1, which was not present pre-operatively.11 The patient underwent a decompression of L5/S1 for SEL, with no signs of disease at three weeks and a five-month follow-up. Another account of rapidly progressive SEL was reported by Youn et al. in a 67-year-old man. He underwent endoscopic posterior foraminotomy at L4-S1. One month later, the patient re-developed leg pain. MRI showed epidural fat posterior to L5. Lipomatosis was removed via endoscopic posterior decompression at L3-L5 and his symptoms resolved.13 These cases are unique as SEL developed acutely after spine surgery.
Similarly, Choi et al. reported a case of SEL after surgery for symptomatic spondylolisthesis.12 The patient had a laminectomy with herniated disk removal at L3/L4 and anterior lumbar fusion at L5/S1. He had a preoperative BMI of 25.5 and only two treatments with epidural steroids before the operation. During the procedure, there were no signs of excess epidural fat. The patient had complete pain resolution after surgery; however, five months later, he developed symptoms of back pain, radiating leg pain, and claudication. MRI showed extensive epidural lipomatosis of the lumbosacral spine, constrictive compression of the thecal sac, and a complete block below L4/L5. After debulking the epidural fat, his symptoms improved. Choi et al. also documented a case of SEL following percutaneous vertebroplasty at T11 and L2.12 The patient’s BMI were only 22.5, with normal basal hormone levels. He had received one dose of epidural steroids one month postoperatively. The patient re-presented five months later with symptomatic SEL. MRI demonstrated compression fractures of L3 and L5 and extensive lipomatosis encasing the thecal sac in the lumbosacral spine. MR myelography showed a narrowing of L5/S1.12
Idiopathic
SEL may develop as a result of idiopathic disease. However, the definition of idiopathic is controversial. Some authors define idiopathic to suggest unknown origins, while others have used it to indicate disease caused by obesity. However, most authors refer to idiopathic as SEL in non-obese patients with unknown cause of origin. Using this definition, literature has shown that 17% of SEL cases are idiopathic, indicating a need for further research to identify more plausible inducers of SEL.26 One study reported aberrant lipid metabolism to be related to idiopathic SEL.54 However, these patients were also obese, again highlighting the controversial definition of idiopathic disease.26
While not an established risk factor, concomitant spinal neurosarcoidosis with SEL has been reported.55 Authors postulate a possible link in pathogenesis due to inflammatory factors such as TNF-α and IL-1β in both diseases.
Diagnosis
The diagnosis of SEL can be challenging given that symptoms often resemble other common etiologies such as vertebral and disc disease. Given this overlap, diagnosis requires a high degree of clinical suspicion and various diagnostic modalities. The most sensitive test is advanced imaging, specifically T1-weighted magnetic resonance imaging (MRI).56 Historically, the “Y” sign or polygonal deformations of the dural sac have been specific for SEL. These pathognomonic signs on MRI are caused by thecal sac compression due to excess epidural fat.17,57,58 However, recent studies have indicated that the “Y” sign does not become apparent until the disease has progressed to severe stages. One specific study found a lower rate of “Y” signs in symptomatic patients compared to the initial research conducted by Kuhn et al., bringing into question the sensitivity of this feature on imaging.58 In a study conducted by Borré et al., grading patterns of SEL were defined based on the epidural fat (EF) to spinal column (Spi C) index, with normal being ≤40% and grading of SEL being described as Grade I, II, or III. Based on this study, Grade III, characterized by an EF/Spi C index of ≥75%, is when the “Y” and other pathognomonic dural sac deformations are first seen on MRI.17
Treatment and Management Introduction and Overview
Given the varied pathogenesis underlying SEL, management requires a unique approach to each patient.4,8 Treatment typically begins conservatively; however, one study found that around 90% of patients will eventually undergo surgery.1 For those who do not respond appropriately to conservative management or suffer from severe symptoms, decompression and/or laminectomy are indicated. Outcomes of surgery depend on the spine level at which SEL is present and whether the etiology is idiopathic or secondary. Specifically, patients with idiopathic SEL at the thoracic level were noted to have the best recovery compared to secondary etiologies such as endogenous steroid use or obesity.1,59,60
Conservative and Medical Management
Weight reduction should be pursued for obese patients, regardless of underlying etiology, as studies have shown improvement of symptoms irrespective of the cause.1,54,61 The degree of weight reduction for symptomatic improvement varies from patient to patient, although one study recommended a 15-kilogram reduction to improve SEL-related symptoms.18 More aggressive weight reduction methods, such as bariatric surgery and calorie-restricted diets, have also been noted to provide clinical improvement due to significant loss of epidural fat deposition.62 Overall, weight reduction may reduce the likelihood of failed management and avoid the need for surgical intervention.63
For patients on chronic corticosteroids, management is directed at a tapered corticosteroid reduction while ensuring adequate treatment of the underlying condition. However, doses as low as 15 mg per day for as little as four months have resulted in patients developing SEL; thus, simply reducing steroid doses will not prevent SEL.64 For certain patients, increasing disease-modifying medications or utilizing alternative therapies in addition to a steroid reduction has led to symptomatic improvement.24
Management of SEL caused by endogenous steroid production depends on the exact etiology and requires a careful workup.1,29 Determining whether the condition is ACTH-dependent or independent is a critical first step for patients with Cushing’s disease. ACTH-dependent patients may benefit from pituitary surgery. If an ectopic ACTH-producing tumor is discovered, removal should improve symptoms. Other alternatives include medical therapy, specifically inhibitors of steroidogenesis such as metyrapone or ketoconazole.41 For patients with hypothyroidism, management is via supplementation with exogenous thyroid hormone. The overarching aim is to treat the underlying condition.
The use of epidural steroid injection as treatment remains a controversial discussion. Most studies oppose the use of steroid injection since steroids are involved in the pathogenesis of the disease, while others report successful pain management.16
Therapeutic Endoscopic and Minimally Invasive Intervention
Minimally invasive procedures are an effective option in treating SEL when conservative measures fail.65–68 Endoscopic removal of fat can be performed similarly to common endoscopic spinal surgeries such as removing a herniated nucleus pulposus.66 Minimally invasive spinal surgeries are preferred due to lower postoperative pain, shorter rehabilitation time, and less cosmetic damage when compared to more invasive surgeries.69
The first reported endoscopic procedure for SEL was performed in 1998 in a 53-year-old male patient with bilateral lower extremity pain and weakness and lower back pain.68 The patient had undergone an L3 decompressive laminectomy ten years prior, but the procedure had not successfully relieved his pain. Evaluation of the lumbar spine with MRI revealed epidural lipomatosis from spinal levels L4 to S1. Decompression of the accumulated epidural fat was accomplished using a malleable endoscopic suction device. The author reported a much more minor surgical defect due to the endoscopic procedure. In addition, no postoperative complications were encountered, and the patient reported no back or lower extremity pain at the 6-month follow-up.68
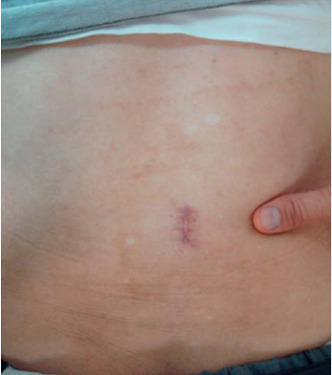
In 2008 Sairyo et al. reported on a 71-year-old male with no prior steroid use who complained of low back and bilateral lower extremity pain, especially while standing upright. Physical and neurological examinations suggested radiculopathy of bilateral L4 nerve roots. MRI scans showed epidural fat compressing the dural sac posteriorly at L3-L4.66 Right-sided L3-L4 laminotomy. Subsequently, subsequent decompression of fat was performed endoscopically, completely removing the fatty tissue hypertrophied ligamentum flavum at L3-L4. Following surgery, the patient could walk upright and reported no lower extremity or lower back pain. No postoperative complications were reported. The surgical scar left by the endoscopic procedure measured 2.0 cm (Figure 1).66
Kang et al. reported three successful cases using a percutaneous bi-portal endoscopic technique (PBES) as a novel treatment of SEL.65 MRI scans before surgery revealed dural sac compression and ligamentum flavum hypertrophy in all three cases. PBES required two 0.7 cm skin incisions superficial to the interlaminar space. An endoscope was placed in the top portal while the necessary tools for laminotomy were guided through the lower portal (Figure 2).65 The procedure involved endoscopically guided laminotomy and ligamentum flavectomy with subsequent decompression of fat with pituitary forceps. The procedure can be performed on multiple levels of the spine during a single procedure. This method was used on a 62-year-old female with SEL compression at L4-L5. A two-level PBES was performed at levels L4-L5 and L5-S1. No postoperative complications were reported, and the patient showed improved quality of life three days after surgery. The authors also reported a successful four-level PBES on a 78-year-old male. This patient had SEL at L2-L5 on MRI scans. PBES at L2-L3, L3-L4, L4-L5, and L5-S1 was performed. Three days post-operation, MRI scans showed spinal decompression at all four levels. A third successful use of PBES was performed on a 46-year-old female diagnosed with L5-S1 epidural lipomatosis. The patient underwent a two-level PBES procedure at L4-L5 and L5-S1 with no complications and improved quality of life postoperatively.65
In 2020 Roberti et al. presented a novel case where minimally invasive tubular laminectomy was performed to drain and remove a cystic epidural lipomatosis.67 The male patient presented with bilateral lower extremity weakness and bilateral sciatica. MRI scans indicated dural sac compression by what appeared to be a cystic mass at L4-L5 (Figure 3).67 The patient elected to be treated with a minimally invasive tubular laminectomy with drainage and removal of the cystic mass. The authors reported a fatty cystic lesion with no clear capsule that was filled with yellow fluid. Pathological investigation of the cystic mass found the specimen to contain fibroadipose tissue with fat necrosis, cystic degeneration, and histiocytic inflammation. No other cases of cystic epidural lipomatosis such as this have been reported in the literature. The patient reported no complications six months post-operation.67
Surgical Management
Large-scale, controlled studies comparing the efficacies of conservative treatment, minimally invasive surgical treatment, and open surgical treatments of spinal epidural lipomatosis have not been conducted due to the condition’s rarity. However, many cases showing the effectiveness of surgical management of SEL have been reported, making surgical intervention a relevant option in the treatment.27,30,56,60,62,70–74 Although, efficacy varies based on underlying etiology. Fogel et al. reported a success rate of 77% with surgery for patients who developed SEL due to exogenous steroids, while Borré et al. reported a 75% success rate.17,26 In the obese population, surgery provided relief to 67%; however, weight loss and conservative management led to a greater success rate of 82%.26 For those with idiopathic disease, surgery led to symptomatic improvement in 21 out of 22 patients.56
In 2016, Ferlic et al. published an analysis of 22 patient-reported outcomes of surgical spinal decompression as a treatment for MRI confirmed lumbar SEL.56 The authors used the Core Outcome Measures Index (COMI) to assess pre-and postoperative pain, function, well-being, quality of life (QOL), work disability, and social disability reported by the patients. After the 3-month postoperative COMI questionnaires, the authors reported a significant improvement (p<.05) in mean (n=22) scores for overall COMI, leg pain, and back pain. While 81% of the patients saw improvement in their COMI scores, only 50% of patients had improved their score more than the minimum clinically significant change (MCIC) of 2.2 points. The significantly enhanced mean COMI score remained at one year and two years (n=20) post-operation compared to the preoperative COMI values. Since half the patients met the MCIC improvement in COMI scores and 81% of patients showing improvement in their COMI score, the authors report decompression surgery to be a clinically relevant option for the treatment of lumbar SEL.56
Yasuda et al. analyzed 16 patients that underwent decompression surgery as a treatment for SEL, with the efficacy of the treatment measured using the Japanese Orthopaedic Association (JOA) scores at 3, 6, and 12 months post-operation.27 The authors reported no recurrent or worsening cases upon follow-up, with a mean (n=16) JOA scores improving from the preoperative value of 15.2 ± 2.8 to 21.3 ± 2.2 after three months, 24.6 ± 1.2 after six months, and 25.4 ± 2.5 twelve months after surgery.
In a review of SEL among the Korean population, Yoo et al. reported 23 cases of SEL where laminectomy with decompression was performed. The authors reported outcomes of “excellent to good” in 22 of the 23 cases, with one case resulting in mortality related to a different medical complication.72 Ferlic et al., Yasuda et al., and Yoo et al. conclude in their studies that surgical decompression is an effective treatment option for spinal SEL only after conservative treatments such as weight loss are proven to be ineffective.27,56,72
Weight loss induced by bariatric surgery has been shown in at least one case to resolve SEL.62 Valcarenghi et al. reported a 48-year-old male with a Body Mass Index (BMI) of 37.4 with MRI confirmed SEL at level L5-S1. The patient was not an ideal candidate for decompression surgery due to his age and weight, so a more conservative approach of a sleeve gastroplasty was performed. Seven months after the procedure, MRI scans revealed almost complete resolution of the SEL.62 Since regaining the weight is often seen long-term in bariatric surgery patients, further studies on the effects of weight regain on SEL treated with weight loss need to be conducted to make bariatric surgery a more clinically relevant treatment option for SEL.75
Since 2018 three publications have compared the outcomes of spinal decompression surgeries between patients with SEL and patients with non-SEL-induced spinal stenosis.60,73,74 Bayerl et al. conducted a three-year observational study comparing the decompression surgical outcomes of patients with SEL (n=38) and patients with classic spinal stenosis (CSS) (n=51).73 On follow-up, patients in both groups were evaluated using the Roland-Morris Disability Questionnaire, Oswestry Disability Index, Numeric Pain Scale, walking distance, and the Short Form-36 form. The authors report a comparable clinical outcome between the SEL and CSS groups at three years post-operation. During the three-year follow-up, no significant differences in any outcome scores were found between the two groups, along with 71% and 69% of patients in the SEL and CSS groups, respectively, reporting satisfaction with the surgery.73
Similar studies have reported findings inconsistent with Bayerl et al.60,74 Fujita et al. analyzed the spinal decompression surgery outcomes of patients with SEL and compared them with patients without SEL.60 All patients were assessed using the Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ) and the Roland-Morris Disability Questionnaire (RDQ). Using a method described in the publication, the authors further classified the surgical outcome as either “effective” or “not effective” during the follow-up visits. They found all but the psychological disorder domain JOABPEQ scores significantly improved in both groups at the 1- and 2-year post-operation visits compared to the preoperative values. Both groups also saw significant improvement in their RDQ scores following their operation. However, the authors’ analysis found that SEL was significantly associated with a “not effective” outcome in 1-year post-op walking ability and social life domains, as well as 2-year post-op walking ability.
Ulrich et al. compared spinal decompression surgical outcomes of patients with SEL (n=14) to spinal stenosis patients without SEL at 1- and 2-year follow-up.74 Pain, disability, and quality of life were assessed using Spinal Stenosis Measure (SSM) symptoms, SSM function, and EQ-5D-3L summary index (SI). The authors found that while disability was significantly improved in the patients with SEL at the 2-year post-op visit, pain and quality of life were not significantly improved. Ulrich et al. concluded that the SEL group had a lower quality of life and more reported pain than those without SEL.74
Han et al. reported a novel surgical method involving internal fixation and bone graft for treatment of SEL in a 53-year-old male with a BMI of 33.3. He presented with low back pain, bilateral leg pain, and numbness. MRI showed severe lumbar spinal stenosis with hypertrophic adipose tissue and ligaments. He underwent decompression, resection of epidural adipose tissue by bipolar cautery, internal fixation, and bone graft fusion. Internal fixation and bone graft fusion help maintain the stability of vertebrae. It has been performed in obese patients with lumbar degenerative disease; however, this was the first time it has been documented as being performed in the setting of obesity-induced SEL.76 At 22 months, the patient had improved symptoms. This may be a feasible technique for SEL in appropriate cases.
Conclusion
Spinal Epidural Lipomatosis is a rare condition caused by excess adipose tissue in the spinal canal. The excess tissue compresses the spinal cord, leading to pain and neurologic deficits. The pathogenesis is largely unknown; however, five major risk factors have been outlined, the most common involving exogenous steroids and obesity. Diagnosis is typically made through MRI and management heavily relies on reducing any factor associated with the disease. It is most often tailored to the patient. In more severe cases, minimally invasive and open surgery has proven to be successful. While the etiology of the disease is becoming more clear, further research is needed for novel treatments and more precise practice guidelines.
Author Contributions
Sherman and Kaye conceived the topic of interest and were involved in planning and supervision. Walker performed a comprehensive review of the literature and drafted the framework. Walker, Stark, Brennan, Smith, Sherman, and Kaye contributed to the writing and editing of the manuscript.