Introduction
Enhanced Recovery After Surgery (ERAS) protocols have standardized approaches to perioperative care. ERAS protocols are multi-modal, evidence-based methods aimed at improving patient outcomes and satisfaction after surgery.1 The goals of ERAS include decreasing patient stress response, length of hospital stay, surgical complications and recovery time back to baseline function.1 Moreover, with growing concern regarding the epidemic of opioid use, abuse and misuse and associated morbidity and mortality, finding opioid-sparing methods for arthroscopic surgeries have become of increasing importance.1,2
Arthroscopic shoulder surgeries can be performed under regional or general anesthesia. Several studies have credited regional anesthesia as having several benefits over general anesthesia for shoulder surgery. These advantages include higher patient acceptance, appropriate muscle relaxation, less blood loss, shorter hospital stay, reduced post-operative analgesia requirements and avoidance of the risks and side effects associated with general anesthesia.2–5 In addition to improving these outcomes, the use of brachial plexus blocks for upper extremity surgeries have been associated with lower post-operative pain scores and perioperative opioid consumption.2,6 One such brachial plexus block is the interscalene block, which is applied to the level of C6 vertebral body between the anterior and middle scalene. This block covers most of the brachial plexus derived from C8-T1 and spares the ulnar nerve.7 Ropivacaine is a long-acting amide local anesthetic commonly used in this block, however its median duration of analgesia is 11.8 hours.8 To extend the analgesic effect, some practitioners use a continuous perineural catheter to allow the local anesthetic to infuse over the course of three days.9,10 In an attempt to prolong the duration of this block without using continuous perineural catheters to avoid risk of infection, multiple adjuvants have been explored through case studies.11–15 One of these adjuvants is dexamethasone, which has been effective in prolonging anesthesia for up to four days and reducing pain medication consumption when used in a peripheral nerve block.12,14,15 A similar effect is seen when using dexmedetomidine as an adjunct.14–17
To improve ERAS strategies in operations of the upper extremity and minimize post-operative pain and opioid use, this study aimed to investigate whether the adjuvant combination of dexamethasone and dexmedetomidine (Dex-Dex) could synergistically provide a longer duration of block and provide superior surgical pain relief compared to using Ropivocaine alone in a perineural local anesthetic administered by interscalene block, and whether reduced opiate doses will be required in consequences.
Methods
Patient selection and study design
Data were collected from 2 different hospitals, one ambulatory surgery center, and 3 different anesthesiologists. A retrospective review was conducted on patients mainly undergoing arthroscopic rotator cuff repairs. Participants were included if they were consenting adults 18 years or older with an ASA 3 or lower as a classification. This classification was used to select for relatively healthy adults. Participants were excluded from the study if they had prior contraindications to nerve blocks such as, infection at the site or sepsis, anticoagulation, preexisting peripheral neuropathy, or an uncooperative patient. IRB approval was obtained for this study prior to beginning patient selection.
Regional Blocks
The patients selected were categorized into two groups: control and treatment (“dex-dex”) group. Patients in the control group received 20 ml of 0.5% ropivacaine. The treatment group received 20 ml of 0.2% ropivacaine along with 5mg of preservative-free dexamethasone and 25 mg of dexmedetomidine as the combined treatment. All patients were placed in a semi-sitting position preoperatively, and a low frequency ultrasound probe was used to identify the brachial plexus using a traditional interscalene nerve block technique, in a sterile manner. All procedures were performed by a board-certified anesthesiologist. A single-shot echogenic block needle was used to administer 20 ml local anesthetic.
Outcome Measurement
Each patient was called at 1-week intervals for 2 weeks and asked about the duration of the nerve block, pain score, and opioid use. Control arm group typically preferred shorter duration blocks whereas the treatment group wanted longer acting pain relief. We also assessed for any complications after the procedure and treatment.
Statistical Analysis
Statistical measures and significance were calculated using Microsoft Excel 365 MSO Version 16.0.13530.20054 (32-bit), and R version 4.0.3 (2020-10-10) “Bunny-Wunnies Freak Out”18, running on Windows 10 version 1903 (OS build 18362.778). A two-tailed Wilcoxon t-test was used to compare means between two groups. χ2 tests were used to infer the significance of the relationship between discrete and non-discrete variables. Standard errors of the mean were computed as accepted. Kaplan-Meier estimates were used to assess the length of regional block activity. A Cox multivariate analysis was run to identify possible confounding variables. P-values<0.05 were considered significant.
Results
A total of 31 patients were eligible for inclusion in the study, of which 19 received pre-operative regional nerve blocks with dexmedetomidine and dexamethasone (“dex-dex”), and 12 served as control, receiving regional blocks with 0.5% ropivacaine. Within the control group, 3/12 patients (25%) received an intravenous (IV) dose of dexamethasone 4mg, while 9/12 (75%) received no dexamethasone; no patients in the dex-dex group received IV dexamethasone intra-operatively.
The two groups did not defer significantly in demographic and baseline conditions (Table 1). The mean age was 55.1 years (median 57.5, range 37-69) in the control group and 52.4 (53, 33-73) in the dex-dex group (p=0.53). There were 6 women (50%) in control group versus 11 (57.9%) in the dex-dex group (p=0.95). In the control group 3/12 (25%) were African American (AA), 1/12 (8.33%) was Hispanic, and 8/12 (66.7%) were White; in the dex-dex group those numbers were 8/19 (42.1%), 1/19 (5.26%), and 10/19 (52.6%) respectively (p=0.62). The average body mass index (BMI) in the control group was 27.83kg/m2 (median 27.5, range 21-44). Average BMI was slightly higher in the dex-dex group at 29.68kg/m2, likely due to a larger range of weights (27, 21-49, p=0.49). Comorbidities were similar between the groups; 3/12 (25%) patients in the control group had hypertension versus 4/19 (21.1%) in the dex-dex group (p=1), 1/12 (8.33%) had dyslipidemia versus 2/19 (10.5%, p=1), and 1/12 (8.33%) were hypothyroid while none were in the dex-dex group (p=0.81). Only one patient in our study was previously diagnosed with chronic pain and belonged to the dex-dex group in this trial (1/19, 5.26%, p=1). A multivariate analysis attempted to identify specific confounders, but was not able to find any significance, either (Table 4).
The patients in this trial were offered regional pre-operatively for similar procedures between the groups (p=0.7). Most of the patients underwent arthroscopic rotator cuff repair, including 11/12 (91.7%) in the control group versus 16/19 (84.2%) in the dex-dex group. A smaller group underwent reverse total shoulder repair (1/12, 8.33% versus 2/19, 10.5%). A single patient was seen for an open repair and internal fixation (ORIF) of a proximal humerus fracture (1/19 in the dex-dex group, 5.26%).
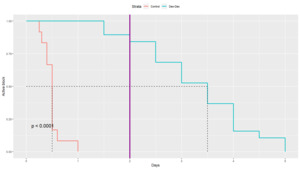
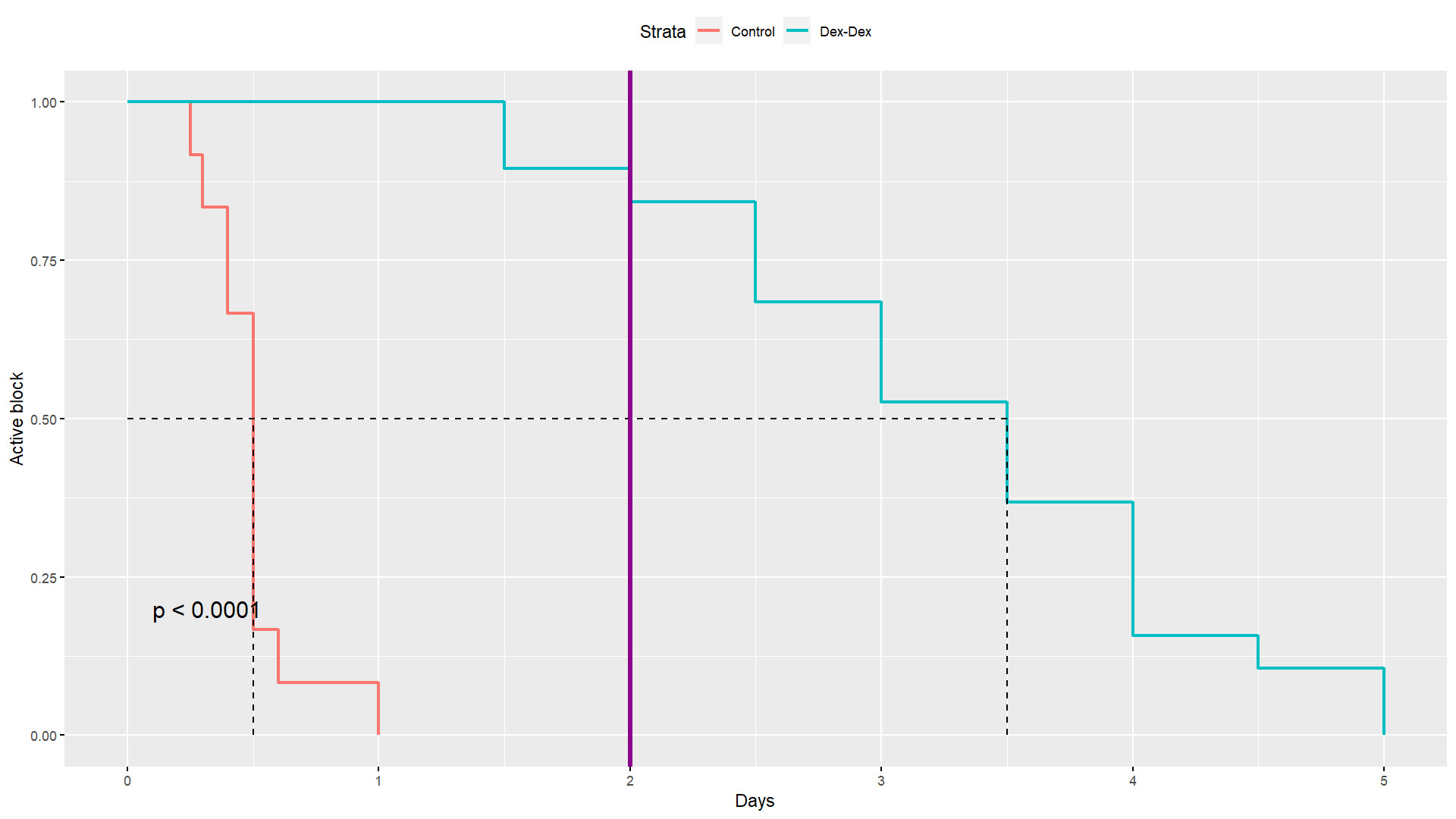
Overall, patients in the dex-dex group had a significantly longer active regional block and experienced longer analgesia from their nerve block (Table 2). The mean time to block termination was 0.5 days in the control group, versus 3.3 days in the dex-dex group (p<0.0001). The medians were 0.5 and 3.5, respectively, and the ranges were disjoint, 0.25-1 days in the control group versus 1.5-5 days in the dex-dex group. A Kaplan-Meier (KM) analysis of block duration was performed to further evaluate the difference (Figure 1); the median duration was 0.5 days in the control group, versus 3.5 days in the dex-dex group, and the overall comparison was highly significant (p<0.0001). Two days post-operatively, 16/19 (84.2%) patients in the dex-dex group still experienced analgesia from the block, while none did in the control group.
Opioid use was also significantly higher in the control group (Table 2); patients in the control group required a mean 36.75 doses of 5mg oxycodone in the post-operative period (183.75mg oxycodone, 275.63mg morphine-equivalent) versus only 14.42 doses (72.1mg, MME 108.16mg) in the dex-dex group (p<0.0001). A violin plot (Figure 2) represents this comparison and further demonstrates the difference not only in mean use, but the distribution as well; the median MME in the control group was 300mg with a 180-300mg range, whereas it was only 82.5mg in the dex-dex group with an 82.5-300mg range.
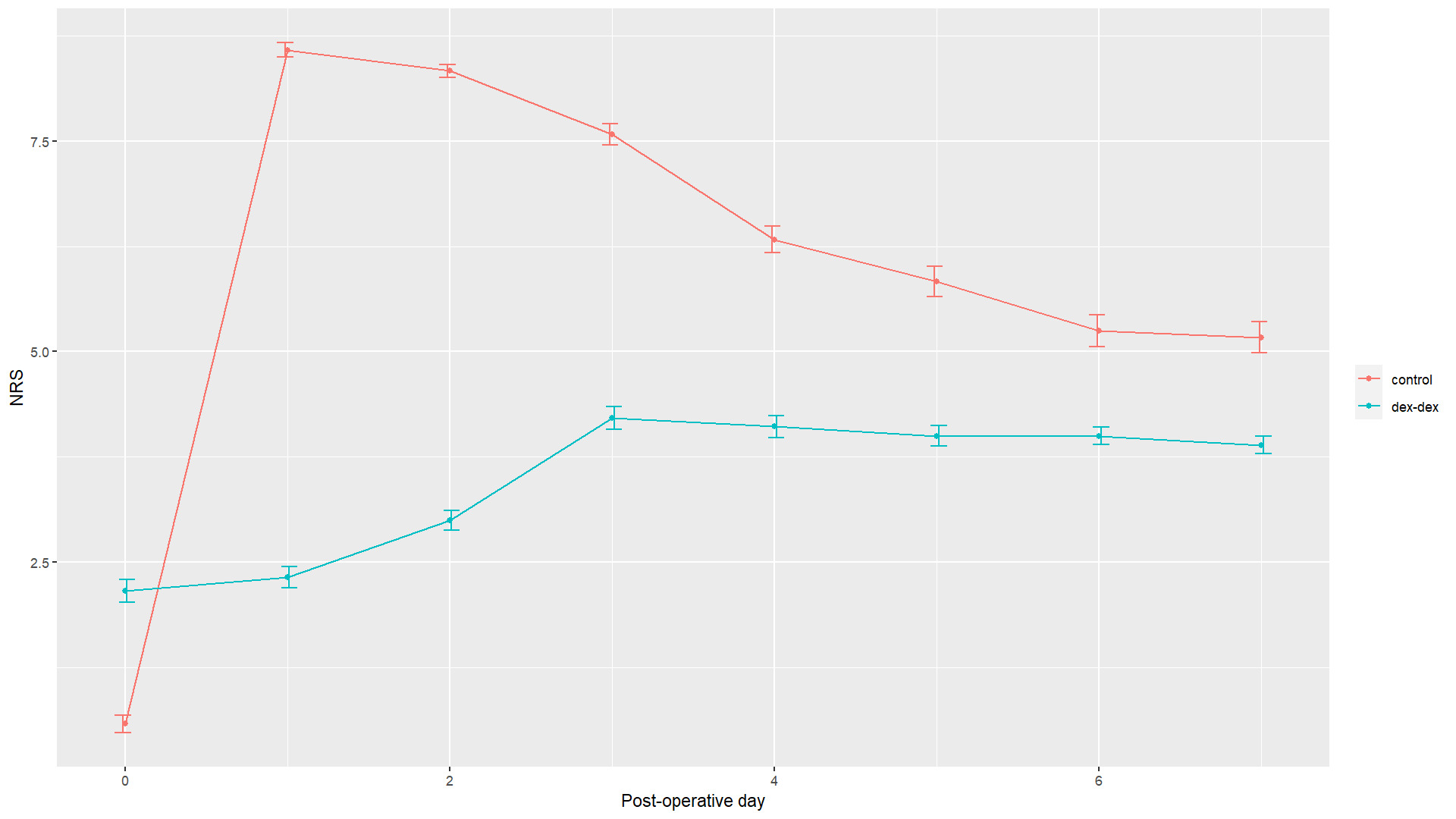
In terms of daily pain relief, patients in the control group demonstrated significantly better pain control immediately following surgery (NRS 0.583 versus 2.16 in dex-dex group, p=0.03). However, patients in the dex-dex group had significantly better pain control in the subsequent days, with significantly lower NRS in days 1-5 post-operatively (Table 3). Though the difference trended towards lower pain in the dex-dex group on post-operative days 6 and 7, that difference was not significant. The maximum average NRS in the control group was 8.58 on post-operative day 1, and 4.21 in the dex-dex group on post-operative day 3. Figure 3 demonstrates the kinetics of pain control between the groups.
There was a single patient who suffered from an adverse event in the dex-dex group; the patient received an interscalene nerve block for a rotate cuff repair. This patient sustained bradycardia, hypotension, and nausea about 20 min after placement of the block, which ultimately resolved. They also described paresthesia persisting for 14 days in the ipsilateral thumb and index finger. No adverse events were recorded in the control group.
Discussion
Shoulder surgery can either be performed under general anesthesia, regional anesthesia, or a combination of both. Often times these practitioners and patients opt for an interscalene nerve block and general anesthesia. The benefit of these techniques are to reduce pain intraoperatively and postoperatively. However, traditional interscalene nerve blocks provide a limited duration of analgesia. Liposomal bupivacaine was developed to increase the duration of the regional anesthesia. In this trial, the combination of dexamethasone and dexmedetomidine as adjuvants to local anesthetics was explored.
The United States, and much of the world, are facing an epidemic of opioid addiction; post-operative and chronic pain prescription are frequently a gateway to opioid use. With up to 30% of Americans suffering from chronic pain, and up to 44% of chronic pain patients prescribed opiate medications for pain control, these numbers are at an all-time high. In fact, in 2016-2017 it is estimated that 76 million Americans were prescribed opiates for pain control, and up to 12% reported misuse of these prescriptions.19–21 Opioid addiction is difficult to abandon, relapse rates are high, and the ultimate price is often paid by the suffering individuals; in 2017 alone, there were 47,506 opioid deaths in the United States.22
In dealing with the opioid crisis, alternative methods for pain control should be a priority for operative, and especially post-operative pain. Though regional nerve blocks have been previously described and provide good operative and post-operative analgesia, they normally last only in the immediate post-operative day. Furthermore, the abrupt cessation of analgesia prevents the gradual adaptation to pain, and often leads to immediate and possibly increased opioid consumption.23
The combination of dexamethasone and dexmedetomidine was previously described and multiple sources quote increased benefit in combination with local anesthetics, suggesting a synergistic effect.16,24–26 The mechanism by which this synergism is driven is largely unknown. Dexmedetomidine, an α2 receptor agonist, is known to provide analgesia through spinal, supraspinal, and peripheral actions. Most likely via activated cation currents and membrane hyperpolarization, dexmedetomidine prolongs the action of local anesthetics when instilled locally. Dexamethasone carries an anti-inflammatory action, inhibits prostaglandin formation and promotes the release of endorphins. The details of their synergistic effects are yet to be discovered.24–26
Prolongation of action of nerve blocks employed for post-operative pain control would likely provide not only improved patient experience but is also likely to contribute to decreased utilization of other analgesics for post-operative pain, namely opioids, as seen in prior studies where dex-dex nerve block facilitated the complete weaning from opioids in patient who had been using them for a prolonged period of time.17
In this study, dex-dex injectate significantly prolonged anesthetic length compared to ropivacaine injectate. This led to a significant increase in mean block length from 0.5 days using 0.5% ropivacaine, to 3.3 days using our dex-dex mixture, with blocks lasting up to 5 days (p<0.0001). Not only that, but on every postoperative day until day 5 after surgery, patients receiving the dex-dex nerve block had significantly less pain than their control counterparts. An important observation seen in Figure 3, is that pain increased gradually in the dex-dex group, compared with the abrupt increase in pain in the control group, likely allowing for better adaptation to the expected post-operative pain. It is, however, unclear why patients in the dex-dex group experienced more pain immediately postoperatively.
By lengthening the analgesic time of the nerve block and adapting the kinetics as demonstrated by Figure 3, the dex-dex group was able to significantly decrease opioid consumption compared to the control group. The mean morphine milligram equivalents used by the control group were 275.63mg, and only 108.16mg by the dex-dex group (p<0.0001). This difference is demonstrated in Figure 2. This could be explained by the length of the block, the kinetics of analgesia, or the combination of both.
Some evidence has emerged to suggest that the use of IV dexamethasone could be equivalent to the use of local instillation of steroids during a nerve block. This study, however, was not powered to evaluate this comparison. Of note, in the 12 patients in our control group, 3 received IV dexamethasone, without significant effect. In fact, these three patients had a block that lasted 0.5-1 days, and all used the maximum 300mg of MME in opioids.
Dexamethasone and dexmedetomidine each carry a risk for side effects on administration. While the side effects of dexamethasone are related mostly to prolonged use, dexmedetomidine can cause decreased cardiac output, bradycardia, hypotension, sedation, and confusion. One of the patients in the dex-dex group experienced transient hypotension, that resolved prior to surgery. The other 18 patients exhibited no side effects of their received nerve block. A prolonged nerve block could also be less desired by some patients, such in the same patient who described paresthesia lasting up to 14 days from nerve blockade.
This study is not without limitations. Though attempts to eliminate confounding factors were taken, the groups in this study were small and the type of study is retrospective in nature. Future prospective trials are needed to reaffirm these results. Larger studies are required to evaluate for adverse events, especially rare events.
Conclusion
In this study, the combination of dexmedetomidine and preservative-free dexamethasone as adjuvants to ropivicaine have demonstrated an increase in duration of pain control, minimizing opioid control following shoulder surgery compared to ropivicaine alone. Future prospective, randomized control trials are needed to confirm these findings. If proven to be effective, such adjuvants may lead to more robust and effective and perioperative ERAS protocols.
Disclosures
The Authors have nothing to disclose.
Funding
No external funding source was used for the generation of this publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.