INTRODUCTION
Periprosthetic joint infection (PJI) represents a relatively rare but severe complication of total knee arthroplasties (TKA),1 with life-threatening consequences and high costs for patients and the healthcare system.2 Debridement of the infected tissues associated with implant removal or not represents the best treatment option facing PJI.3–7 Moreover, radical debridement of all necrotic tissues, synovectomy, combined with replacement of mobile prosthetic parts, and systemic antibiotic treatment, namely DAIR, eight is reported to be effective in those patients affected acute postoperative implant infections. Early postoperative infection is usually defined as occurring within four weeks from index arthroplasty, although some authors consider any infection within three months (90 days) from the index arthroplasty as acute.8 Conversely, hematogenous infections are often classified as ‘late infections’ since they can occur up to two years after joint arthroplasty, caused by blood spread of bacteria from a distant primary focus that can secondarily affect the joint space.9–13 Although hematogenous PJIs characterized by immature bacterial biofilm can be effectively treated with DAIR, as acute infections, the results in terms of infection eradication rate are highly variable.
This systematic review of literature aims to I) evaluate demographic data and microbiological findings of patients affected by hematogenous periprosthetic knee infections II) clarify the effectiveness of the DAIR procedure in treating hematogenous periprosthetic knee infections.
MATERIALS AND METHODS
Registration
Before commencing the review, the protocol was registered online with PROSPERO (International Prospective Register of Systematic Reviews) (reg. prot. CRD42018064291).
Data sources and search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.14 Electronic databases, namely MEDLINE, Scopus, and Scholar, were reviewed for studies investigating DAIR treatment in hematogenous (late acute) knee periprosthetic infections. A combination of the following keywords was used for article search: “PJI OR periprosthetic joint infection OR Prosthetic infection” AND “acute hematogenous infection” AND “debridement OR implant retention OR DAIR” AND “knee OR knee joint” AND “treatment outcome”.
Eligibility criteria
The inclusion criteria were not limited to English literature and specific publication dates. Reference lists of selected articles were searched for additional pieces that were not identified in the database search. In addition, longitudinal studies (retrospective and prospective) evaluated DAIR’s treatment outcome in hematogenous knee PJI were included. The exclusion criteria included: case reports, expert opinions, previous systematic reviews, letters to the editor.
Furthermore, we excluded: (I) studies that do not perform only open debridement (II) and studies that included different joints involved in which knee data could not be extrapolated (III) papers which did not report joint age, and within these, patient with a joint age inferior to 3 months.
Study assessment and data extraction
Initially, the titles and abstracts of the studies were screened by two independent reviewers (RdG, EF). Next, the full text was obtained for all the abstracts that appeared to meet the inclusion criteria or those with any uncertainty. Then, each study was assessed based on the inclusion criteria by two independent reviewers. Finally, any disagreement regarding the inclusion of any study was resolved by evaluating the article by the senior Author (GB).
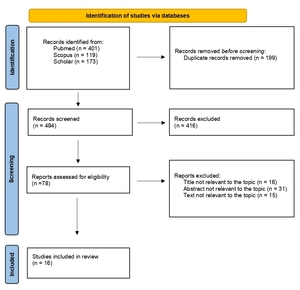
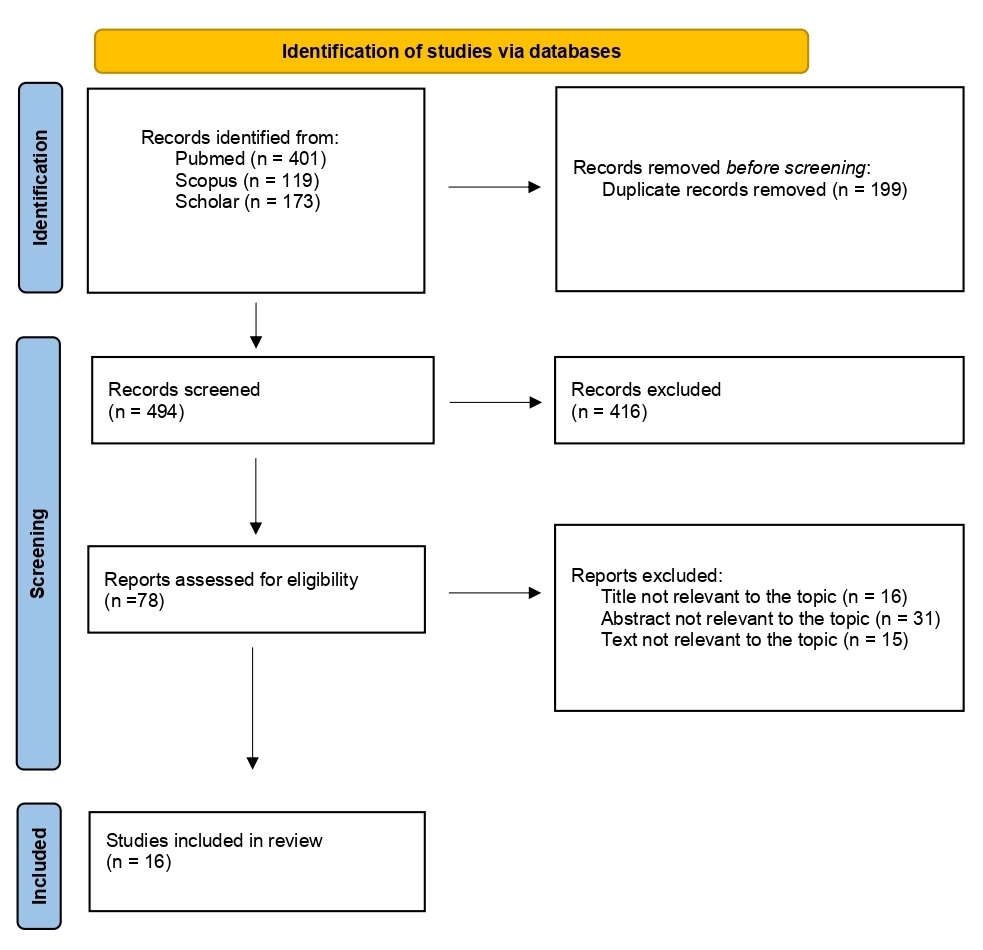
The flow diagram of our search strategy is presented in Figure 1. A total of 693 potentially relevant studies were found through computer search and manual screening of reference lists. Having removed all duplicates, 494 articles remained for evaluation. After screening the titles and abstracts, 416 studies were excluded, and full texts of 78 articles were evaluated and screened for relevance to the review’s topic. In total, 478 studies were excluded after a detailed assessment. The remaining 16 articles were included in our systematic review.
Relevant data were extracted from each included study. In addition, data describing participants’ demographics, follow-up, microbiology, diagnosis classification, treatment options, and outcomes were collected and recorded.
RESULTS
Sixteen papers met the inclusion criteria for a total of 430 infected knee implants (Table 1). Based on reported data in 13 of the included studies,1,2,10,15–25 hematogenous knee infections have occurred in patients with a mean age at diagnosis ranging from 56 years old,8 to 74 years old.24,25 Only six studies described the percentage of males among the patients treated,15,17,18,21,24,25 ranging from 18%,8 to 66%.18 The mean follow-up varied from 27 months,19 to 98 months.20
In stratifying the diagnosis of infection, the most used classifications were the one proposed by Tsukayama et al.,26 in 10 out of 16 (62,5%), followed by Zimmerli-Tramputz et al.,27 in 3 out of 16 (18,75%) (Table 1). Fever was reported as the first symptom by Vilchez et al. in up to 83.3% of patients.25
All studies in our systematic review reported the causative microorganisms involved in the septic process (Table 2). Gram-positive species (spp.) were the most represented (86% of the total), with Gram-negative spp. Representing only 8% of the causes of acute hematogenous infections, and other microorganisms such as fungi accounted for less than 1%; culture-negative diseases were reported in 5% of the total analyzed cases.
In all the selected papers, an open debridement as a surgical procedure has been performed. The replacement of mobile parts of the knee implants was described to be completed in a percentage of patients varying from 29%,21 to 100%.1,15,17,22–25,28 Two papers did not mention modular component exchange.2,18
Mean interval time from onset of symptoms to DAIR was reported in all papers, but two,18,22, ranged from 1 to 60 days. Twelve of the included documents treated patients within 28 days from the onset of symptoms.10,15,17,19–21,23–25,28–30 Reported joint age, defined as the time interval between index procedure and DAIR, ranged from 2 weeks to over 20 years (Table 3).29
In ten out of sixteen papers, intravenous (iv) antibiotic therapy for six weeks, followed by a variable therapy per os, was the antibiotic regimen applied.1,10,15,17,18,21,22,24,29,30 The rest of the papers reported to have practiced an iv therapy for between 1,5 to 4 weeks (Table 3).2,19,20,23,25,28 The use of rifampin as antibiotic treatment for staphylococcal PJI was described in only six papers (Table 3).10,15,19,23,25,28
The definition of failure was specified in all papers included. Failure to eradicate infection, a wound fistula, drainage, intolerable pain, or infection recurrence caused by the same organism strain; subsequent removal of any component for disorder; unplanned second wound debridement for the ongoing deep condition; and occurrence of periprosthetic joint infection-related mortality were considered failures of DAIR procedure.31 Conversely, success was defined as retention of implant, with no signs and symptoms of infection and regular laboratory markers after a minimum of 12 months from DAIR.
The percentage of failures ranged from 0%,20 to 69%.29
The main microorganism responsible for the failure was S. aureus, accounting for 36 of the 23 failures in which the involved bacteria were described (Table 3). In case of failure of DAIR treatment, patients generally repeated open debridement (8 on 12 papers)1,17,20,21,23,25,29,30: other options were two-stage revision,2,15,21,23,24,30or suppression antibiotic therapy (SAT).1,2,22
DISCUSSION
The treatment of periprosthetic joint infection represents a challenge for orthopedic surgeons. While the management of chronic diseases has been well described,4–7,16,32 there is a lack of information on the management and outcomes of patients treated for an acute hematogenous infection. To the best of our knowledge, there are no systematic reviews that describe the effectiveness of the DAIR procedure in infection eradication in patients affected by a hematogenous periprosthetic knee infection. Our study shows an average success rate of 41% (31% to 100%), at mean follow-up; this outcome is slightly worse than the moderate DAIR success reported for acute infection by Maillet et al. (51,7%),33 Romanò et al. (45.9%),34 and Vilchez et al. (75% early infection VS 45% hematogenous infections).25 One possible reason for this result may be the lack of a clear definition of acute hematogenous PJI. Two classifications have been proposed to better distinguish acute postoperative and hematogenous infections. Zimmerli and Tramputz, back in 2004, defined as hematogenous PJI any infection that occurs after 24 months from index procedure.27 Instead, Tsukayama et al. defined as hematogenous PJI any deep infection characterized by acute presentation in a previously well-functioning joint arthroplasty.26 In our systematic review, the Tsukayama and Zimmerli-Tramputz definitions are applied in 10 and 3 of included studies.26,27 Another possible reason is the period from symptoms onset to DAIR procedure instead of the time from index procedure (joint age). Our data suggest that the best outcomes are described by authors reporting the shorter period from diagnosis to surgical procedure, with an average of 6,4 days.(1-60)
These results are by the Second International Consensus Meeting (ICM) on PJI,13 that advice to perform a DAIR when the onset of symptoms is <4 weeks (good < 7 days). In addition, according to the Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA),35 patients who have a well-fixed, functioning prosthesis without a sinus tract, infection occurring within 30 days of index arthroplasty or < 3 weeks of the onset of infectious symptoms, and an organism susceptible to oral antimicrobial agents should be candidates for debridement antibiotics and implant retention (DAIR). In our systematic review, the reported joint age, defined as the time interval between index procedure and DAIR, ranged from 2 weeks to 20 years. Health status, such as nicotine abuse, the ASA status, and the number of previous operations represent the leading causes of increased risk to a hematogenous infection even after different years from prosthetic implantation.2,18,28
In the end, how the DAIR is performed and the management of modular prosthetic components may influence the outcome. As reported, 100% of the selected papers have conducted an open debridement. Still, in some cases, the modular part was not exchanged–whereas the ICM stated that exchanging modular components reduces the recurrence of infection.13 Specifically, Choi et al. did not remove the liner in all cases,29 and Estes et al. reused the mobile parts after a sterilization process10; it is remarkable that the worst results were found in the group who did not perform routinely polyethylene exchange.10,29 It has been stated at that same consensus meeting that DAIR may prove a solid therapeutic option in treating acute infections in unicompartmental knee arthroplasties and mega prosthesis alike,13 widening its indication to more than just primary or revision TKA.
Other factors have been confirmed to be associated with treatment failure in acute PJIs treated with DAIR, including the type and virulence of the microorganisms involved and the consequent duration of prescribed antibiotic treatment.30 Polymicrobial infections were the most difficult to treat, showing up to 75% failures. Whenever coagulase-positive staphylococcal spp. has been isolated, cases failed an average of 55%, staphylococcus Epidermidis (50%) followed by Enterococcus spp. (38%) and Streptococcus spp. (37%). Gram-negative bacteria have reported a success rate of nearly 90%, as well as fungi (Table 3). Furthermore, high failure rates were reported in those case series that provided an IV antibiotic duration of fewer than six weeks.2,25
In case of failure of DAIR, many Authors repeated debridement1,17,20,21,23,25,29,30; other options were two-stage revision or lifelong antibiotic suppression therapy. Salvage procedures were performed in selected cases.17,29,30 A repeated DAIR with an interval of 7 days was not reputed a failure. To this matter, the ICM in 2018 relevantly stated that after a failed DAIR procedure, one and two-stage revisions should be considered rather than attempting a second time.13
To the best of our knowledge, ours is the first study focused on DAIR treatment in acute hematogenous periprosthetic infection of the knee, with a mean follow-up of two years and a high number of patients enrolled. Selection criteria resulted in decent uniformity of surgical treatment.
Like every publication, our work has some limitations: despite the strict selection criteria of the surgical treatment, conclusive studies lack homogeneity of medical treatment. The antibiotic treatment scheme was not uniform in duration and molecules used. Furthermore, the database search retrieved no RCT studies.
In conclusion, debridement, antibiotics, and implant retention has been demonstrated to be a safe, economical, and effective technique but have to be performed in a very narrow temporal window; modular component exchange is strongly advised. In addition, further studies are needed concerning the antibiotic treatment (antibiotic choice, duration, and regimen) to reach an effective standardization.
Authors’ contributions
EF, VdM, and ML screened the studies and selected the data. MA, RdG, and TA wrote the manuscript. Finally, GB and MM corrected the whole paper.
Competing interests
No author is involved in a conflict of interest. No author has received any funding.
Funding
No funding source was involved in the conduction of this study.