INTRODUCTION
Tarsal tunnel syndrome (TTS) is caused by compression of the posterior tibial nerve (PTN) and its branches within the tarsal tunnel. The tarsal tunnel is a narrow osteofibrous space in the posteromedial aspect of the ankle formed between the underlying talus, the calcaneus, the posterior portion of the medial malleolus, and the overlying flexor retinaculum.1 This confined area contains some of the important anatomic structures of the distal lower extremity, such as the tibial neurovascular bundle, flexor tendons, and tibialis posterior tendon.2 Various conditions, including obesity, lower extremity edema, inflammation, trauma, space-occupying structures, and hypertrophy of the flexor retinaculum, may cause an increase in the pressure within the tarsal tunnel and compression of the PTN.3 Subsequently, blood flow to the PTN may decrease, resulting in local ischemia. This in turn decreases the ability of nerve cells to transmit action potentials4,5 and gives rise to symptoms and signs such as pain radiating to the heel and the plantar aspect of the foot, numbness, paresthesia, and in severe cases, muscle weakness and atrophy.6,7 The diagnosis of TTS is made primarily based on characteristic clinical findings. Adjunctive tests such as electrophysiological studies and magnetic resonance imaging (MRI) may provide additional information and aid in the differential diagnosis. One of the most common conditions that mimic TTS is plantar fasciitis which also presents with plantar heel pain.2 Furthermore, plantar fasciitis can present concurrently with TTS and advanced imaging such as MRI may allow for better characterization of the plantar fascia that can be released concomitantly in addition to TTR in surgical cases.
Initial management of patients with TTS should be performed nonoperatively with rest, shoe wear modifications, anti-inflammatory medications, physical therapy, anti-neuropathic pain medications, and local injections of anesthetics with corticosteroids guided by ultrasound (US).8,9 Operative treatment, including tarsal tunnel release (TTR), is reserved for patients who do not respond to non-operative treatment methods.8–11
The use of US guidance for injection of anesthetics with corticosteroids locally into the tarsal tunnel provides advantages in terms of accurate needle placement.12 The purpose of this study was to investigate the clinical course of patients with TTS after US-guided tarsal tunnel injections. The study also aimed to compare the demographic characteristics and clinical outcomes of patients who did not undergo TTR with those who underwent TTR after US-guided injections.
MATERIALS AND METHODS
Ethical Considerations
This retrospective comparative study was performed after obtaining approval from the Institutional Review Board for Health Sciences Research. Data collection was performed retrospectively by reviewing computerized patient charts. Hence no written or oral consent from the subjects was required.
Inclusion criteria were as follows: patients diagnosed with TTS based on clinical exam findings including a positive Tinnel’s sign, pain, and paresthesia in the posteromedial ankle and heel that radiates toward the plantar aspect of the foot and the toes, who failed conservative treatment and received US-guided local injections of anesthetics with corticosteroids. Patients with peripheral neuropathies, spinal neurogenic claudication, peripheral vascular diseases, or ischemic claudication were excluded from the study. For every patient, the level of pain was noted on a scale from zero to ten before and after each injection. The patients were divided into non-surgical and surgical groups. The nonsurgical group included the patients who received US-guided tarsal tunnel injections and did not subsequently undergo TTR. The surgical group included those patients who received US-guided injections and ultimately underwent TTR.
The two groups were compared in terms of age, gender, body mass index (BMI), number of injections received, post-injection follow-up time, and the amount of pain reduction immediately after injection (ΔPN). The patients in the surgical group were assessed in terms of clinical outcomes and the presence or absence of additional foot and ankle pathologies that required concomitant procedures in addition to TTR. Although electromyography (EMG) and nerve conduction velocity (NCV) were not routinely performed at our institution due to relatively high false-negative rates and a lack of high-level evidence supporting its use as a diagnostic tool,13 data with regard to EMG and NCV results was also extracted from each group. Additionally, patients who had MRI in both groups were identified. MRI results were assessed in terms of presence or absence of space occupying structures and anomalous muscles or tendons within the tarsal tunnel, and features suggestive of plantar fasciitis.
Ultrasound-guided injection protocol
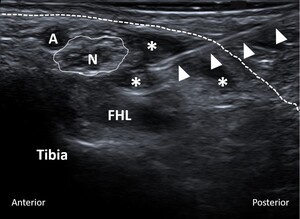
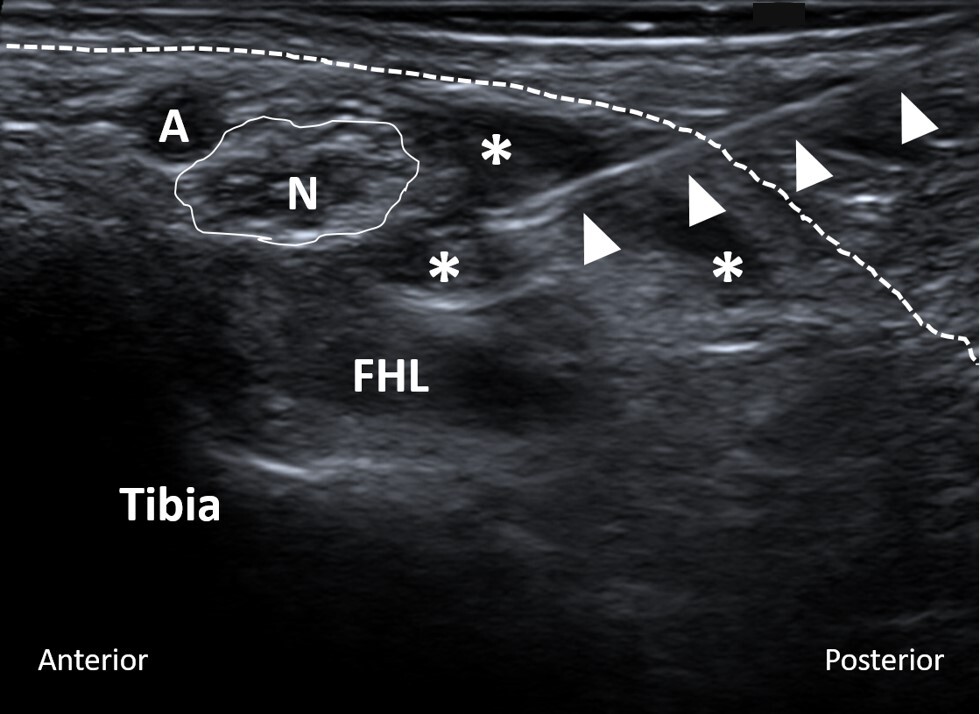
All US-guided tarsal tunnel injections were performed by experienced musculoskeletal radiologists at our institution. The level of pain around the involved foot and tarsal tunnel area before the injection was recorded on a scale from zero to ten. Utilizing local anesthesia and a sterile technique, a 25-gauge needle was advanced into the tarsal tunnel under US guidance (Figure 1). Subsequently, 0.5 cc of 0.25% bupivacaine mixed with 0.5 cc (20 mg) of triamcinolone (40 mg/mL) was injected. After the injection, the level of pain was recorded once again.
Statistical analysis
Descriptive data was generated for both the nonsurgical and surgical groups. Comparisons between the groups were performed using independent t-tests for continuous variables and chi-square (χ2) tests for categorical data. An alpha level of 0.05 was used for all analyses.
RESULTS
There were 218 patients who were diagnosed with TTS and received US-guided injections as a part of their conservative treatment between January 2013 and December 2019 at our institution. All the patients had a positive pathognomonic Tinel’s sign along the tarsal tunnel accompanied by pain and paresthesia in the posteromedial ankle and heel with radiation distally toward the plantar aspect of the foot. After US-guided tarsal tunnel injections, 169 patients (77.5%) did not undergo TTR (nonsurgical group) as a result of improvement in their clinical signs and symptoms. The remaining 49 patients (22.5%) ultimately chose to undergo TTR (surgical group) after injections (Figure 2). There were no complications in either group related to US-guided injections. The average follow-up time after the last injection for the nonsurgical group was 339 (range: 15–1903) days. The average time between the injection and TTR for the surgical group was 145 (range: 15–505) days. Table 1 summarizes patient demographics, study data regarding the number of patients who received more than one injection, and ΔPN for both groups.
In the surgical group, the average follow-up time after TTR was 117 (range: 15–315) days. At the final postoperative follow-up, 41 patients (84%) had complete resolution of pain and neurological symptoms. Eight patients (16%) had either no improvement in the symptoms or reported residual pain and paresthesia. There was no difference between the mean ΔPN values of the patients who had complete resolution of TTS after surgery and those who had reported either no improvement or residual symptoms (3.8 versus 4, respectively; P = .88). Sixteen patients (32%) in the surgical group had additional foot and ankle pathologies that were treated surgically along with TTR. Of the eight patients who did not have complete resolution of symptoms after surgery, three had concomitant procedures performed in addition to TTR. There was no relationship between the resolution of symptoms and additional procedures (P = .74). There were two patients who had complications related to surgical intervention. One patient had numbness around the medial aspect of the calcaneus, and the other patient developed complex regional pain syndrome after surgery. Both complications were detected within the subgroup of patients with additional foot and ankle pathologies that were addressed surgically along with TTR.
Electromyography and NCV studies were performed on 15 (9%) patients in the nonsurgical group and 17 (34%) patients in the surgical group (P < .0001). The EMG results were consistent with the clinical diagnosis of TTS in three (20%) patients in the nonsurgical group and eight (47%) patients in the surgical group (P = .107). In the surgical group, six out of eight patients with positive EMG results and five out of nine patients with negative EMG results had complete resolution of TTS at the final post-surgical follow-up (P = .7).
MRI was obtained in 68 patients (40%) in the nonsurgical group and 27 patients (55%) in the surgical group (P = .064). MRI results demonstrated space-occupying structures in the tarsal tunnel in 1 patient in the nonsurgical group and 4 patients in the surgical group (P = .008). There were MRI findings indicative of plantar fasciitis in 26 patients (38%) in the nonsurgical group and 3 patients (11%) in the surgical group (P = .009). None of the three patients with MRI findings of plantar fasciitis in the surgical group underwent plantar fascia release. At the final post-surgical follow-up, although two patients were symptom-free, the remaining patient still had medial plantar heel pain.
DISCUSSION
Tarsal tunnel syndrome is a relatively uncommon diagnosis, and the literature does not provide a precise estimate with regard to its incidence and prevalence.14 To our knowledge, the current study includes the largest number of patients with TTS who received US-guided injections as a part of their conservative treatment at a single institution. Our results demonstrated that US-guided injection could be an effective nonsurgical treatment option for patients with TTS and should be considered before proceeding with surgical decompression of the tarsal tunnel.
There is consensus in the literature that conservative treatment of TTS should be initiated before surgery.6,8 All patients included in this cohort presented with activity-limiting heel/medial ankle pain and a Tinel’s sign along the tarsal tunnel that had failed conservative measures including stretching, physical therapy, orthotics, and shoe wear modification. Despite a lack of definitive evidence in terms of the real incidence and prevalence of TTS, several studies showed that the majority of patients with TTS respond well to conservative treatment, and surgery is performed in less than 30% of cases.1,15 In a retrospective series including 81 patients with TTS who were evaluated via the US, only 23 patients (28%) underwent surgical TTR.1 Mondelli et al.15 performed surgical releases on 5 of 23 patients (21%) with TTS who had follow-up data available. Similarly, the current study showed that surgery was performed on 22.5% (N = 49) of the patients with a clinical diagnosis of TTS who received US-guided tarsal tunnel injections (N = 219).
An interesting finding of the present study was that the mean age of the patients in the surgical group was significantly lower than that of the patients in the nonsurgical group (48.8 years versus 53.8 years, respectively; P = .03). It could be speculated that younger patients with relatively higher physical activity levels may tend to choose surgical treatment due to higher severity of symptoms and more prominent limitations in daily activities. Notably, there were 3.4 times more females than males in both groups. Women are known to be more susceptible to certain nerve entrapment syndromes, such as carpal tunnel syndrome, as a result of a combination of hormonal and anthropometric factors, such as a greater percentage of body fat content.16 Although TTS has not been studied as extensively as carpal tunnel syndrome, it would be reasonable to suggest that similar factors may play a role in the female predilection for TTS as well.
Supporting the premise that a greater percentage of body fat might be a predisposing factor for nerve entrapment syndromes, average BMI values for both the non-surgical and surgical groups were found to be above the normal range of 18.5 to 25 (29.8 versus 30.04, respectively; P = .88). Furthermore, we calculated the average BMI value for 50 patients who were seen in our outpatient clinic most recently and had no clinical diagnosis of TTS. The average BMI value for the whole study population with TTS (i.e., the combination of both non-surgical and surgical groups) was higher than that for the patients without TTS (29.93 versus 27.37, respectively; P = .01).
It is suggested that local injections of corticosteroids into the tarsal tunnel should be performed carefully because of the potential for tendon rupture and intravascular injection.6,8 All the injections in our study were performed under US by senior radiologists with broad experience in US-guided musculoskeletal injections. Fortunately, no complications were detected during or after the injections in any of the cases. The mean ΔPN scores after the injections were not significantly different between the nonsurgical and surgical groups (3.62 versus 3.88, respectively; P = .053). The amount of pain was graded immediately before and after the injections that included both anesthetics and corticosteroids. As the anesthetic effect is observed instantaneously and short-lived, the longevity of the effects of corticosteroids within the injection has a more definitive role in achieving long-term relief of symptoms. Therefore, the difference in ΔPN between the two groups would probably be more profound if the grading was repeated after a longer period following the injections.
Another point worth discussing is the inclusion of the patients who had additional foot and ankle pathologies that required surgical treatment along with TTR. Sixteen patients had procedures such as hammertoe corrections (1 patient), Morton’s neuroma excision (7 patients), peroneal tendon repair (4 patients), and medial calcaneal displacement osteotomy (1 patient), gastrocnemius recession (2 patient), and secondary Achilles reconstruction (1 patient). Patients with such additional pathologies may elect to undergo surgery to address more than one problem during the same anesthesia session instead of trying extended conservative treatment for longer or receiving further injections into the tarsal tunnel.
There is a lack of consensus with regard to the diagnostic value of EMG for TTS. Since a negative EMG result cannot rule out the diagnosis of TTS, EMG studies are not routinely performed on patients with TTS. Fantino et al.1 studied 81 patients with a clinical diagnosis of TTS, and EMG was performed on 25 of those patients (30%). The results of EMG were positive for TTS for only 13 patients (50%). In the present series, significantly more EMGs were performed in the surgical group than the nonsurgical group (34% versus 9%; P < .0001). This difference can be explained by the resolution of symptoms in the nonsurgical group after US-guided tarsal tunnel injections that obviated the need for EMG studies. Another possible explanation for the performance of more EMGs in the surgical group could be the tendency to obtain further diagnostic studies before proceeding with surgical intervention. The EMG results were positive for 47% of the patients who underwent testing in the surgical group. Furthermore, negative EMG results did not appear to be related to suboptimal outcomes after TTR.
An MRI scan can be valuable for detecting space-occupying lesions as the cause of TTS.6 Moreover, MRI can also be helpful for identifying or ruling out concomitant pathologies such as plantar fasciitis. In our study, MRI was performed in 40% of the nonsurgical group and 55% of the surgical group patients. In the present study, every patient who was clinically diagnosed with TTS received a US-guided injection. Therefore, US imaging of the tarsal tunnel was already performed along with the injections and this alleviated the need for additional imaging. It is worth noting that significantly more patients in the nonsurgical group had MRI findings of plantar fasciitis compared with the surgical group (38% versus 11%, respectively; P = .009). This finding underlines the importance of considering plantar fasciitis in differential diagnosis and identifying it as a pathology that can be found along with TTS. In the surgical group, four patients had space-occupying lesions in the tarsal tunnel including accessory flexor digitorum longus (two patients), ganglion cyst (one patient), and exostosis (one patient). Although MRI can confirm the presence of space-occupying lesions, PTN may appear normal on imaging when no specific focal masses are present in the tarsal tunnel.17 Additional MRI findings such as focal fatty atrophy of the abductor digit minimi, may suggest entrapment of the first branch of the lateral plantar nerve (Baxter’s neuropathy).18 We do not routinely order MRI for evaluation of tarsal tunnel syndrome. Ultrasound imaging during injection into the tarsal tunnel can be sufficient to suggest plantar fascia involvement which occasionally leads to further advanced imaging prior to surgical treatment.
The present study had some weaknesses that are inherent to the retrospective study design. The patients in the nonsurgical group were not followed regularly for a standard time period after the injections, and the outcomes of surgical TTR were not evaluated using standard scoring systems. However, including a large number of patients with TTS, a standard US-guided injection protocol, and having a group of surgically treated patients after injections for comparison were the strengths of this investigation.
CONCLUSION
The current study suggests that TTS is more commonly seen in women. The US-guided injection of anesthetics with corticosteroids into the tarsal tunnel can be a safe and effective nonsurgical treatment option for patients with TTS. Our results indicate that nonsurgical treatment may more commonly fail in younger patients. In patients who fail to respond to nonsurgical treatment, TTR may provide for the complete resolution of signs and symptoms in the majority of the cases.
Authors’ contributions
KA, preparation of the outline, data collection, manuscript writing, preparation of the tables and figures; BS, data collection, manuscript reviewing and editing; JP, preparation of the tables and figures, manuscript reviewing and editing; VP and TC, manuscript writing, reviewing and editing; JP, preparation of the outline, manuscript writing, reviewing and editing.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.

._intraoperative_pictures_of_tarsal_tunnel_release.jpg)
._intraoperative_pictures_of_tarsal_tunnel_release.jpg)
._intraoperative_pictures_of_tarsal_tunnel_release.jpg)
._intraoperative_pictures_of_tarsal_tunnel_release.jpg)