Introduction
Among young people, adolescent idiopathic scoliosis (AIS) is a common cause of spinal deformity. Epidemiologically, AIS is the most common form of scoliosis1,2 with a reported prevalence of 5.2% in Germany.3 In the United States alone, it has been estimated that AIS affects more than 4 million people, with approximately 1 million children exhibiting some degree of spinal deformity.4 In accordance with this epidemiological data, the frequency of spinal fusion has been increasing in some countries.5
While bracing is an effective treatment in some cases,6 progression to more severe AIS is often treated by posterior spinal fusion (PSF).5,7 Bony fusion in PSF is usually achieved by dorsolateral decortication and application of autologous iliac crest bone graft (ICBG),8,9 a technique for which the reported rate of pseudarthrosis is less than 5%.10,11 However, donor site morbidity is an issue of this technique and is reported to occur in 2% to 45% of all cases.12,13 This donor site morbidity is why augmentation by bone graft substitutes (BGS) is becoming more popular than the augmentation by ICBG, for example in short segment fusion and treatment of pseudarthrosis.9,14,15
While data exists for single level and short segment fusions, there presently is a paucity of data on fusion rate after bone augmentation with BGS in multisegmental PSF. The leading concern is pseudarthrosis, which often leads to a loss of correction after PSF.16 Therefore, the bone graft is an essential aspect of PSF. As more segments are involved in the PSF, larger amounts of bone graft are needed, and in paediatric cases the supply of ICBG could pose a problem. Therefore, the use of an effective BGS instead of autologous bone grafts would be beneficial, especially in paediatric deformity surgery where large amounts of bone graft are needed for multisegmental PSF, with a subsequent high rate of donor site morbidity.12,13
ICBG is considered the gold standard for achieving bony fusion, however the use of BGS to support bony fusion is becoming more common.17 While BGS is established for use in short-segment posterior spinal instrumentation, there is insufficient data regarding its use in multisegmental spinal fusion. Therefore, this study was conducted to analyse if a natural BGS leads to a fusion rate that would be comparable to that seen with the use of autologous bone graft after PSF for AIS.
Methods
This was a retrospective review of a prospectively maintained database at our institution. This study was approved by the local ethics committee (Register number 4948) and was conducted according to the revised Declaration of Helsinki. In our clinical documentation system, we identified consecutive patients who were treated operatively for AIS from 02/2008 to 12/2017. We included patients who were treated by selective spinal fusion (SSF),18 and in whose surgery a bovine-derived apatite BGS (Orthoss® Geistlich Pharma AG, Wolhusen, Switzerland) had been used. We excluded patients with incomplete sets of data or a follow up of less than 24 months. Further we excluded patients with other bone substitute, in order to focus this research question as well as to limit the potential variables. During surgery, the BGS was applied as bone augmentation in a 1:1 ratio with local autologous bone graft that was derived from facet joints, laminotomies and rip hump resections.
Bone Graft Substitute
The BGS that we used (Orthoss®) is bovine-derived, yet the topography of this substitute is similar to human bone with a high pore connectivity, while the macroporosity of the xenograft is similar to cancellous bone.19 As a natural hydroxyapatite, there is neither residual organic material nor protein residues, and its potential for bone regeneration has been documented in vitro, via a high permeability to cells and fluids and being rapidly surrounded by new bone without foreign body reaction or encapsulation.20 These conditions indicate that the BGS is stable over time. Its slow resorption thus makes it valuable for indications where bone volume maintenance is important for long-term stability of the heterotopic bony fusion.20 Although the clinical use of this material has been documented as early as 1991,21 the published literature concerning its application in the spine has been limited, with 1 recent paper noting successful outcomes when the material had been used in treatment of vertebral trauma.22
Outcome measures
Using full spine radiographs (FSR), we measured the Cobb angle of the main and the minor curve as well as the preoperative thoracic kyphosis (TK). These were done directly post-operative, and then at 6, 12 and 24 months postoperative. In these FSR, we measured the Cobb angle of the instrumented main curve and, if applicable, the Cobb angle of the instrumented minor curve. Depending on the spinal fusion that was performed, an instrumentation of the minor curve was not performed in all patients. Additionally, we measured the angle of the TK of the instrumented segments of the curve. Radiological signs for screw loosening or breaking were documented, as well as complications such as breaking of rods or revision surgery.
All radiographs were analysed by measuring instruments from the Sectra IDS 7-PACS System (Sectra, Linköping, Sweden). All statistical analyses were performed under R, version 3.6.3, using the coin-package (version 1.3.1) and the asht-package (version 0.9.6).
Results
Using diagnostic codes, we identified 42 patients who had undergone operative treatment for AIS in our institution between 02/2008 and 12/2017. After application of the inclusion and exclusion criteria, 9 patients were excluded because other bone substitutes were applied. Another 5 patients were excluded due to an incomplete follow-up. Therefore, we analysed the data from 28 patients in our investigation. The demographic data is presented in table 1.
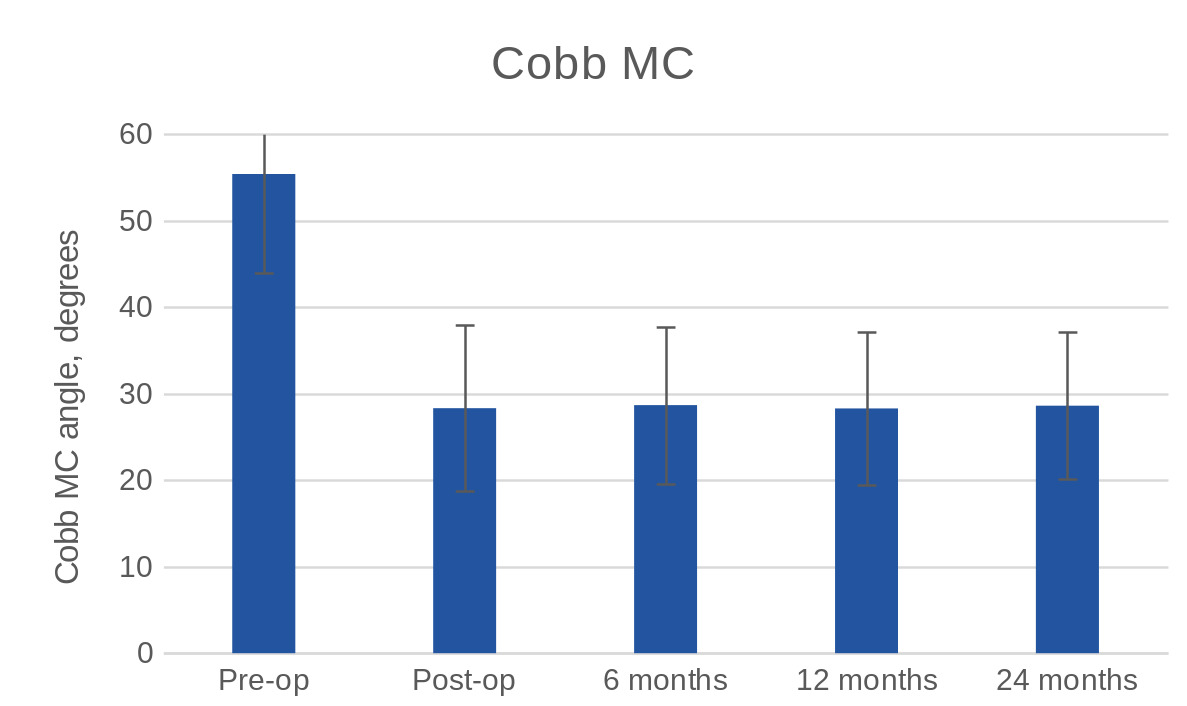
Based on the pre-operative FSR, the average Cobb angle of the main curve was 55.39° ± 11.46° while the average Cobb angle of the minor curve was 35.64° ± 12.79°. The average Cobb angle of the instrumented main curve in the FSR directly postoperative was 28.31° ± 9.56°. Thus, there was a correction of the main curve by 27.07° ± 10.94°, or 48.7%.
At 6 months, the average Cobb angle of the instrumented main curve was 28.67° ± 9.06°. At 12 months control the average Cobb angle of the instrumented main curve was 28.29° ± 8.83°while the exam at 24 months showed that the average Cobb angle of the instrumented main curve was 28.6° ± 8.51°. Maximum loss of correction of the Cobb angle of the main curve during the 24 months of follow up was 3°. There were no significant differences of the Cobb angle between any of the post-operative time points, as shown in Figure 1.
There was an average change of the TK of the instrumented segments in the FSR at the 6 months control of 0.35° ± 1.09° while the analysis at the 12 months control revealed an average change of 0.42° ± 1.53° and the 24-month analysis showed a change of 0.64° ± 1.60°. There were no significant differences in this value when compared between any of the post-operative time points
The loosening of one screw was detected in three patients (10.7%) during the 24-month follow-up. In all 3 cases, it was a screw in the lowest instrumented vertebra (LIV) that had loosened. There was no revision surgery needed in 27 cases (96.43%) while there was 1 case (3.57%) of revision surgery due to a rod fracture following trauma (fall from a swing directly on the back).
Discussion
In this consecutive cohort of 28 patients, we noted no significant changes of either the Cobb angle or the TK angles, as measured from immediately post-operative to the 24-month post-operative follow-up. The maximum deviation of the Cobb angle at the end of follow up was 3° for the TK and for the main curve in the frontal plane it was 3°. In addition, we noted 1 screw loosening in each of 3 patients, therefore we consider that the use of the bovine-derived BGS was part of a surgical treatment plan that resulted in a radiographic success rate of 100%.
The role of bone graft remains an important component of PSF, yet the supply of ICBG may remain a limiting factor in choosing the graft material. In adolescents such as we treated, who had not reached skeletal maturity, there is the question of availability of cancellous bone from the iliac crest, a topic which has led to the investigation of alternate grafting materials.23 A more recent meta-analysis of bone grafts in paediatric patients had also cited bone graft availability as necessitating the consideration of material that could achieve the same goal.24 The results that we have presented lend credence to the hypothesis that the bovine-derived BGS that we implanted during PSF is capable of maintaining the correction, as evidenced by the lack of change in Cobb angle of thoracic kyphosis. The success rate of 100% compares favourably with the outcomes that had been noted in a large systematic review, in which the fusion rate was reported to be 86.4%.25 Additionally, our fusion rate was comparable to the recent paper by Weber, et al., (2020) in which the same BGS was used and the authors reported radiographic evidence of fusion in 83% of the levels treated.22
We choose an increase of the Cobb angle of 5° as threshold for a relevant progression of the curve during the 24 months of follow-up and as a possible indicator for a missing bonny fusion because of data from other study groups. Pruijs, et al. (1994) had reported that the standard deviation of the differences in Cobb angle for repeated measurements of different investigators was 3.2 degrees and for the repeated measurements by one investigator, it was 2.0 degrees.26 Peterson and Nachemson defined Curve deformity progression >5° as failure of treatment.27 None of our patients exceeded this threshold. Thus, there was no loss of correction as an indicator for missing bony fusion in any of our patients.
We detected a screw loosening in 3 of our patients. However, every time a screw was diagnosed as loosened, it was a loosening of a screw from the lowest instrumented vertebra. LIV was in the apex of the minor curve in all 3 patients and thus related to inadequate selection of LIV,28 and not to missing bony fusion, especially since there was no intention to achieve bony fusion below the LIV.
It is known that screw loosening is an indicator for a missing bony fusion.29
With regard to other assessments of failure, we had to perform revision surgery in 1 of our patients. However, the patient needed a revision due to trauma that happened between the 12 and 24-month follow-up. As a result of this accident, the trauma the patient suffered a break of one rod. Thus, in our opinion revision surgery in this case could not be used as an indicator for a missing bony fusion.
Our results for multisegmental PSF are comparable with previous data for short segment PSF. For example, Garin, et al., (2016) reported no fusion failures after the use of the same BGS in PSF. However, their patients underwent PSF for an Average of 4 segments.30 Korovessis, et al., (2005) had also presented comparable results for bony fusion when a coralline BGS was used for a 1 or 2 segment PSF.9
While the outcomes that we have reported are generally positive and suggest that this particular BGS can play a role in surgical treatment for AIS, there are limitations to this study. While a notable limitation of our investigation is that we performed a retrospective data analysis, the data that we presented are routinely acquired in all patients and we enrolled consecutive patients, thus it reflects real-world experience. A further limitation may be that we only looked for indirect markers of a missing bony fusion. Examples for indirect markers are the loss of deformity correction in the frontal or sagittal profile, measured by postoperative changes in the Cobb angle of the instrumented regions, radiological signs for screw loosening in the x-ray or an increase of pain.31,32 These indirect markers have been shown to be effective in identifying a missing bony fusion after a 24 month follow up.31 As a direct marker, bone fusion can be identified in a CT scan,33 but use of a CT (in this case for follow-up) would have led to higher doses of radiation for our patients and there is published data indicating that a cumulative, effective dose of radiographs correlates with a higher incidence of breast cancer in scoliosis patients.34 Therefore, we prefer to limit the use of CT scans in patients with AIS.
Conclusion
In our cohort of consecutive patients, we noted a fusion success rate of 100%, as evidenced by the maintenance of the Cobb angle as well as maintenance of the reduced thoracic kyphosis. As well, we noted the loosening of 1 screw in each of 3 patients in the LIV with no other treatment failures. The data indicates that the use of this BGS during PSF can provide an effective adjunct to autologous bone grafts in adolescent patients.
List of Abbreviations
AIS adolescent idiopathic scoliosis
PSF posterior spinal fusion
ICBG iliac crest bone graft
BGS bone graft substitutes
SSF selective spinal fusion
FSR full spine radiographs
TK thoracic kyphosis
LIV lowest instrumented vertebra
Acknowledgements
We would like to acknowledge Dr. Paul Tregouet for his editorial assistance in the preparation of this manuscript and Udo Wittmann from consult ag – statistical services for performing the statistical analysis
Funding
The author(s) disclosed no receipt of financial support for the research, authorship, and/or publication of this article
Conflicts of interest/Competing interests
MRK reports personal fees from Globus Medical, outside the submitted work.
The other authors declare no conflict of interest relevant to this work.
Availability of data and material (data transparency)
The datasets generated during and / or analyzed during the current study are not publicly available due data protection but are available from the corresponding author on reasonable request.
Code availability (software application or custom code)
Not applicable
Authors’ contributions
MP: Conception and design, Administrative support, Collection and assembly of data, Provision of study materials or patients, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
JW: Administrative support, Provision of study materials or patients, Manuscript writing, Final approval of manuscript.
MRK: Conception and design, Administrative support, Provision of study materials or patients, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Ethics approval
This study was approved by the local ethics committee (Register number 4948) and was conducted according to the revised declaration of Helsinki
Consent to participate
Not applicable because of the retrospective design of the investigation
Consent for publication
Not applicable because of the retrospective design of the investigation
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication