Introduction
Aseptic loosening, instability, and infection are the major causes of knee prosthetic joint failure, often necessitating revision arthroplasty.1 The differential diagnosis between septic and aseptic prosthetic loosening is critical due to the different treatment approaches and often very difficult.2–5 Clinical presentations of periprosthetic infection are extremely varied ranging from acute/high grade to subclinical/low-grade prosthetic joint infection (PJI) and biofilm-related implant malfunction.4,6 Moreover, laboratory tests yield nonspecific or conflicting results. The definition of PJI has often been debated and several diagnostic criteria have been proposed over the years.2,3,5 Generally, joint aspiration with culture is mandatory for a correct diagnosis. Nevertheless, even if microbiology testing is specific, its sensitivity is variable. Moreover, also biopsies and cultures from joint aspiration expose to the risk of infection. Clinical presentation and common laboratory tests for inflammation (erythrocyte sedimentation rate (ESR), C reactive protein, blood leukocyte count, and pro-calcitonin) are non-specific for the diagnosis of periprosthetic joint infections (PJI). Recently, promising results have been reported regarding synovial biomarkers tests, including the alpha defensin immunoassay and synovial fluid CRP tests.7,8
Nowadays, the best strategy to achieve a correct diagnosis derives from the combination of clinical, laboratory, microbiology with imaging techniques.9 Ultrasonography can be performed early and can be used to evaluate periprosthetic fluid collections, attempting to differentiate abscesses from aseptic collections, and guiding a needle biopsy if appropriate.2 X-ray examinations are the standard examination to perform after arthroplasty and for the follow-up, assessing the presence of displacement, mobilization of the implant components, periprosthetic bone resorption, and other causes of pain. However, periprosthetic bone abnormalities are usually non-specific for infection. In addition, up to 50% of conventional X-ray exams give false-negative results.2 Computed tomography (CT) or magnetic resonance (MR) imaging shows the extent of bone and/or soft-tissue involvement. In particular, MR has been shown to be highly sensitive (92%) and specific (99%) for diagnosing PJI in patients with knee arthroplasty, and has the advantage of not using ionizing radiation or contrast agents.10 Nevertheless, both CT and MR imaging accuracy is limited by metal artifacts.10
This review focused on the role of nuclear medicine imaging in knee prosthetic joint loosening, analyzing all the different techniques available.
Materials and methods
A pubmed sarch was conducted using “arthroplasty”, “knee prosthesis”, “infections”, “mobilization”, “bone scintigraphy”, “labeled leukocyte”, “FDG PET”, “positron emission tomography”, in various combination by selecting studies over the last 10 years.
Three phases bone scintigraphy
Three phases bone scintigraphy (TPBS) is the first nuclear medicine imaging method that could be used in suspected knee prosthetic joint failure because widely available, easily performed, and cost-effective.9,11 Bone scintigraphy is usually performed with Tecnetium 99 metastable (99mTc) methylene diphosphonate which accumulates on the surface of the bone mineral matrix, in relation to the blood flow and the osteoblastic activity. TPBS consists of the dynamic imaging sequence called as “blood flow” or “perfusion phase” or “first phase” assessing the perfusion on the region of interest, the early static images of at 3-5 minutes from the radiopharmaceutical injection, known as the “blood pool” or “second phase”, evaluating the relative vascularity, and the late images known as “osteotropic phase” or “third phase”, acquired after 2-4 hours, showing the bone turnover.9
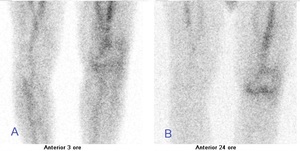
Increased tracer uptake in all of the three phases indicates the presence of an inflammation process and can be observed in PJI (figure 1). TPBS is characterized by an elevated negative predictive value,12 so an alternative cause of periprosthetic joint pain should be investigated in case of negative bone scintigraphy. However, in a positive scan, the differential diagnosis between PJI and aseptic joint prosthetic loosening is a challenge. A more focal and more evident in the late images uptake is described in aseptic joint prosthetic loosening, with respect to the intense and wider uptake, extended also around the prosthesis, often observed in the case of infection both in the early and late images.9,13 However, it is not a specific pattern. TPBS has high sensitivity (93%) but poor specificity (56%) for knee PJI as demonstrated in a systematic review and meta-analysis of Verberne and colleagues.14 Besides, Ouyang et al. found lower diagnostic performance in knee than in hip PJI.15 Therefore, the value of TPBS in knee PJI assessment is expressed by its negative predictive value (NPV) ranged from 96% to 100%.12,16
Moreover, it is useful to remember that many other conditions could be associated with increased uptake of bone scan (figure 1), like fractures and fissures, especially in the uncemented prosthesis, or heterotopic ossification, even if more common in hip prosthesis.17 Besides, a remodeling process is more evident for bio-inductive prostheses.17 Abscess or hematoma, usually identified on CT scan, sometimes can be visualized on the blood flow phase without a corresponding higher bone uptake in the late phase of TPBS.17 The finding of a “hot patella” defined as increased tracer uptake in the patella, higher than the ipsilateral distal femur or the proximal tibia, is a relatively frequent finding on the third phase of the bone scan. It is associated with prevalent anterior and movement pain and can be caused by patellofemoral arthropathy (mainly in a bicompartmental knee prosthesis), patellar instability, patella fractures, or patellar insertion mobilization. Also, osteoarthritis or intraosseous engorgement pain syndrome, that had venous stasis or increased pressures in the bone marrow near the painful joint, could cause this scintigraphic pattern. Patients with “hot patella” who underwent secondary patellar resurfacing had symptomatic relief of symptoms, suggesting a benefit in patients with clinically defined anterior knee pain.10,17
By integrating a tomographic acquisition with single-photon emission computed tomography (SPECT) combined with computed tomography (CT) technique, better sensitivity and specificity than TPBS is demonstrated in the diagnosis of aseptic loosening and periprosthetic infection in patients with painful knee arthroplasty.18 SPECT/CT increased diagnostic accuracy, identifying alternative causes of pain than TPBS alone,18–20 suggesting to integrate this diagnostic tool in the clinical practice.
There is no unambiguous agreement on timing after surgery to perform a TPBS imaging. Periprosthetic activity around knee arthroplasties is also present in asymptomatic patients, in more than 50% of femoral components and nearly 90% of tibial components more than one year following implantation.2 Even if periprosthetic uptake generally decreased over time, there is considerable patient-to-patient variation, and a bone scan, especially in the ostheotropic phase, may be positive for at least 5 years after total knee arthroplasty (TKA) due to physiological bone remodeling.9,13 Consequently, positive bone scans should be interpreted with caution during that period. On the other hand, a negative bone scan excludes a PJI even within the above reported time windows.21,22
Scintigraphy with radiolabeled leukocyte
Leukocyte labeling is performed with indium-111 (111-In) oxine or, more frequently, with 99mTc hexamethylpropylene amine oxine (99mTc-HMPAO), characterized by higher imaging quality with a more favorable radioactive profile for the patient. This technique foresees blood sampling, with subsequent isolation of white blood cells (WBC) which are labeled and re-injected to the patient.9,22,23 The majority of leukocytes labeled are neutrophils. Strict aseptic conditions are required for the procedure and simultaneous labeling of WBC from multiple patients is discouraged to prevent possible cross-contamination. During the processing, care should be taken that leucocytes are not damaged, as this would result in leakage of the radioactivity from the cells, adhesion of labeled leukocytes to the vascular endothelium (especially in the microvasculature of the lungs), and loss of motility. To avoid degradation of the radiopharmaceutical and radiation damage to labeled cells, 99mTc-HMPAO-labeled WBC should be reinjected as soon as possible, not later than 1 h after labeling.24 Unfortunately, labeled leukocyte scintigraphy is a time-consuming procedure, with a complex labeling process, and a high cost. Therefore, it is not performed in all nuclear medicine centers.
Considering 99mTc-HMPAO-labeled leukocytes, images should be acquired at three different time points with decay time-corrected acquisition: “early images” (within 30 min and 1 h after radiopharmaceutical injection), “delayed images” and “late images” (at 2-4 h and 20-24 h after radiopharmaceutical injection, respectively).23,25 Diagnosis of a PJI is made on planar images, qualitatively assessing the increase in uptake or size between the delayed and late images (figure 2). By contrast, the reduction of labeled WBC accumulation over time is interpreted as a sterile inflammatory process.25 Heterotopic ossification, metastatic disease, and degenerative arthritis does not accumulate labeled WBC.24 The quantitative evaluation by calculating the target-to-background ratio (TBR), expressed as a ratio between radioactivity in the suspected region and background area, improves diagnostic accuracy when qualitative assessment is doubtful.23 Glaudemans et al.,26 indicated the specular region of the target area on the contralateral side as the most accurate for assessing the background activity. There is no consensus of a cut-off value of percent increase to distinguish a PJI but is suggested that an increase of TBR at least 10% is considered a reliable indicator of granulocyte accumulation over time,26 even if the positive predictive value (PPV) increase with the increasing of percentage threshold.24 The issue of whether ongoing antibiotic treatment may interfere with the diagnostic accuracy of WBC scintigraphy has been widely disputed. There is not sufficient evidence in the literature to reach a definitive conclusion on the impact of antibiotic treatment on the accuracy of WBC scintigraphy in PJI. In general, some studies demonstrated the reduction of diagnostic accuracy in research of infection focus, while some other found no differences,23 as recently demonstrated by a large multicentric study of 168 patients.27 Anyway, the discontinuation of antibiotic therapy must be agreed upon in a multidisciplinary context in relation to the clinical status of the patient.23 Tomographic images are recommended in case of positive planar images to assess the exact location and extent of the infectious process.2
This imaging technique is considered the gold standard nuclear imaging for the diagnosis of infections in the bone and soft tissue, except for spondylodiscitis.23,25,28 The sensitivity, specificity, and accuracy of the exam differ from different techniques and interpretation modalities (e.g. decay time-corrected or fixed time acquisition, qualitative or quantitative evaluation). Considering only decay time-corrected acquisition protocols, Glaudemans et al.28 demonstrated a sensitivity of 85.7%, a specificity of 93.6%, a diagnostic accuracy of 92.6%, NPV of 97.8%, and a PPV of 66.7% in 54 patients with knee prosthesis infection. Similar results are reported in Erba et al. considering 15 patients with suspected knee PJI (sensitivity 93%, specificity 100%, accuracy 98%, NPV 93%, and PPV 100%).23 By adding SPECT/CT, the diagnostic accuracy increases, thanks to the better distinction between bone infection and soft tissue infection, by evaluating the extent of the infection.23,29
The great disadvantage of leukocyte scintigraphy is that leukocytes accumulate not only in the infected area but also in the bone marrow.22 To avoid radiotracer uptake by reticuloendothelial cells or fixed macrophages of the marrow, bone marrow imaging is performed with 99mTc-sulfur colloid or 99mTc-nanocolloid. Concordant findings between both techniques rule out an infectious process while discordant findings (uptake on labeled WBC scintigraphy without corresponding uptake on bone marrow scintigraphy) are highly suggestive of an infection. The combination of labeled leukocyte/bone marrow scintigraphy appears the best available imaging technique to detect infection in patients with suspicious PJI due to the increase of specificity.30 The reported accuracy of the use of combined WBC/bone marrow scintigraphy ranges from 83% to 98% for both hip and knee prosthesis infections,23 with reported average values of sensitivity and specificity of 70.57% and 94.6% respectively,22 even in recently implanted prosthesis.31
Anti-Granulocyte Antibody Scintigraphy
The acquisition protocol is similar to labeled WBC scintigraphy, with registration at 16–24 h after injection, and performing an early scan if required. Radiolabeled WBC and mAbs imaging are comparable. In some studies32,33 mAbs have specificity slightly lower than radiolabeled WBC scintigraphy. Conversely, in Verberne and colleagues14 anti-granulocyte scintigraphy resulted more specific than leukocyte scintigraphy. However, they are registered in Europe only for osteomyelitis diagnosis and besilesomab, being a murine-derived antibody, may induce human murine antibodies (HAMA) in the hosts, so this radiopharmaceutical cannot be used in the follow-up.25
FDG PET/CT
The role of 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) in diagnosing infections and inflammation is now well established.34 Activated leukocytes demonstrate increased expression of glucose transporters with a higher affinity for FDG. PET/CT offers several advantages over traditional procedures, with higher spatial resolution, higher TBR, and less time required to perform the exam.35 However, FDG accumulates at sites of inflammation but is not specific for infections. Moreover, the presence of metal devices can create reconstructive artifacts, due to metallic interference with CT scan.
Studies about the utility of FDG PET/CT in PJI show conflicting results.34 According to the meta-analysis performed by Kwee et al. the pooled sensitivity and specificity for the diagnosis of hip or knee PJI is 82.1% and 86.6%, respectively.36 Studies reported better performance for hip than knee prosthesis (pooled sensitivity 86% vs. 70% and pooled specificity 93% vs. 84%, respectively)14,37 and also a better accuracy (89.5% vs. 77.8% respectively).38
Comparing with labeled WBC scintigraphy, Love et al., found a higher diagnostic accuracy of combined l 111In-labeled WBC and 99mTc-sulphur colloid scintigraphy than FDG PET/CT.39 In Verberne et al. FDG PET/CT resulted less specific than anti-granulocyte scintigraphy (84% vs. 95%, respectively) and combined labeled leukocyte/bone marrow scintigraphy (84% vs. 93%).14 Likewise, Vanquickenborne et al.40 reported similar sensitivity between the two imaging tools (88%), but higher specificity for WBC scan than FDG-PET (100% vs. 78%). Also, Van Acker et al.41 found sensitivity, specificity, and PPV of 73%, 100%, and 60% for FDG PET/CT and of 93%, 100%, and 83% respectively for the combination of TPBS/labeled WBC scintigraphy. Conversely, Pill et al.42 reported higher sensitivity and specificity for FDG-PET/CT (95% and 93%, respectively) compared to combined 111In-labeled WBC and 99mTc-sulphur colloid (50% and 95%, respectively). Delank et al. demonstrated a sensitivity of 100% for septic cases and of 45.5% for aseptic inflammation of FDG PET/CT imaging in hip and knee prosthesis. However, the authors point out as the reliable differentiation between abrasion-induced and bacteria-caused inflammation was not possible using 18F-FDG-PET.43
Discussion: diagnostic process
A painful prosthesis must always lead to suspicion of infection. The correct diagnostic and therapeutic approaches are now increasingly multidisciplinary, as the combination of clinical, laboratory, and imaging evaluation is required. Table 1 reported the International Consensus Meeting (ICM) of Philadelphia in 2018 criteria for PJI.44
Most recently, World Association against Infection in Orthopaedics and Trauma (WAIOT) proposed another definition of PJI,4,22 identifying “rule out” and “rule in” features, including for the first time imaging tools (Table 2). In particular, due to the high NPV, a negative TPBS is sufficient to “rule out” infection while the WBC scintigraphy is considered as a “rule in” test, in relation to its very high specificity for identifying periprosthetic joint infection versus aseptic loosening. Alternatively to labeled WBC, mAbs could be used, especially to integrate diagnostic algorithms in subclinical infection. To ascertain the FDG PET/CT value more studies are needed. There is a general agreement of its highly sensitive and therefore the high negative predictive value, while is debated its specificity.
Diagnosis of acute infection is rather uncomplicated. Furthermore, some patients showed obvious clinical signs of infection or diagnostic aspiration. The most challenging diagnostic situation is in the case of subclinical presentation with persistent pain after surgery (more than 6-12 months as usual) and/or with persistently slightly elevated inflammation indices values. A periprosthetic infection that goes unrecognized will lead to the failure of a revision arthroplasty for loosening.23
Exactly in this broader interpretative context, nuclear medicine imaging could help differentiate PJI from aseptic mobilization. If the situation requires a very urgent screening test, particularly in patients with a low pre-test probability of infection, we can perform a bone scan or FDG PET/CT (both able to effectively rule out a PJI when negative). However, both TPBS and FDG PET/CT show low specificity, and residual inflammation could be detectable for a long time after surgery. In the case of positive TBPS/FDG PET/CT, or if there is a high suspicion of infection, also in a recent prosthesis implant surgery, it is preferable to perform a labeled WBC scan. Figure 3 is represented the diagnostic flow-chart proposed by EANM, EBJIS, and ESR (with ESCMID endorsement) consensus of 2019.22 Anyway, a common decision-making process is still not validated.
Conclusions
Nuclear medicine imaging is a useful diagnostic tool of knee PJI, which adds functional information to clinical-laboratory evaluation and morphological studies. However, as described, integration within a multidisciplinary diagnostic process is mandatory for proper diagnosis, which involves collaboration between orthopedists, infectious disease specialists, radiologists and nuclear medicine physicians.
Authors contribution
Michele Boero: validation, writing—review and editing.
Michela Allocca: writing—original draft preparation, data curation.
Nicola Pisu, Silvia Sanna, Alessia Ruggiero, Bi Llie .Joy Pung, Simone Margotti: data curation, visualization.
Giuseppe Dessì: Conceptualization, Supervision.
Disclosure
Nothing to disclose.


_consensus_of_2019_diagnostic_flow-chart.jpeg)


_consensus_of_2019_diagnostic_flow-chart.jpeg)