INTRODUCTION
Ewing Sarcoma (ES) is an aggressive tumor that disproportionately affects children, adolescents, and young adults. It is the second most common primary bone malignancy in this demographic behind osteosarcoma.1 The standard treatment for newly diagnosed localized ES includes chemotherapy treatment with ifosfamide and etoposide (IE) alternating with vincristine, doxorubicin, and cyclophosphamide (VDC) every two weeks for the chemotherapeutic duration.2,3 Following chemotherapy, local control by means of a complete surgical resection or radiation therapy is necessary.2,4,5 The 5-year survival rate of localized ES is 81%, whereas metastasized ES has a much poorer 5-year survival rate of 38%.6
With rare neoplasms such as ES, both access to care and proficiency in treating the specific malignancy may affect patient outcomes. Patients living in rural areas may be disadvantaged by the distance required to travel to a specialty referral center with sufficient experience in treating rare bone cancers like sarcomas. The relative lack of patient volume presenting with sarcomas in rural regions compared to metro regions could lead to differences in experience treating ES, which may adversely affect patient outcomes. High-volume cancer centers performing treatments that require specialized surgical intervention have been shown to attain more favorable outcomes in both bone and non-bone cancers.7–10
Variability in cancer mortality rates has also been shown to be true when examining location of care.11 Metropolitan regions tend to have more favorable outcomes in the treatment of common cancers, however these areas do have higher death rates for certain cancers, including stomach, liver, and uterine.11 There is currently a lack of analysis on how geographical factors might affect patients with rarer cancers such as primary bone malignancies. Recent research has found that rural patients have diminished overall survival in osteosarcoma cases.12 This study aims to determine whether ES patients in rural areas are subject to similarly adverse outcomes. We hypothesis that patients in rural counties, as defined by population size and adjacency to a metropolitan area, have worse ES outcomes as measured by tumor size at presentation, time to treat, and survival rate.
METHODS
Study Design and Database
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database was retrospectively queried for primary ES of bone from 2005-2019 in patients less than or equal to 25 years of age. The SEER Stat program was used to generate a case listing of all patients meeting this criterion.
Variables
Exogenous variables of interest included race and origin, sex, median county income, age, county rurality, and year of diagnosis. Outcome variables included tumor size as measured by largest diameter in centimeters, time from diagnosis to treatment in months, months of survival, and cause-specific mortality. Exogenous variables were compared between rural and metropolitan using a two-tailed T-test. Only patients with data in all variables were used for analysis.
Statistical Analysis
To conduct the regressions, a generalized linear model (GLM) was employed. A Poisson model with log link function was employed and controlled for race, sex, median county income, and age to determine the association between non-metropolitan county and tumor size. A similar model with a Gamma distribution and log link function was created for the association between non-metropolitan city and time to treatment. Coefficients were interpreted as log ratios.
To compare survival, a multivariate Cox Proportional Hazard Model with controls for age, race, gender, income, and tumor size, stratified by age and income, was utilized to determine the impact of rurality on survival. To aid in clinical interpretability of these results, the estimated survival for a metropolitan and rural patient was constructed, with covariates set to the median value across the dataset.
Significance level was set to alpha=0.05 a priori. All statistical analysis was conducted using Python using the Pandas, statsmodels, lifelines, NumPy, and Plotly packages. The Python program was written in Visual Studio Code (Microsoft Corporation, Redmond WA) and run using a Docker container (Docker Incorporated, Palo Alto, CA).
RESULTS
Demographics
Across the dataset, a total of 951 metropolitan cases and 155 nonmetropolitan cases of Ewing sarcoma were recorded in the study period. There were 7 metropolitan and 4 non-metropolitan cases excluded due to incomplete data. After eligibility criteria was applied, 868 patients were analyzed, with a mean age of 14.14 years. Of these, 97 patients lived in rural counties (11.18%). Income breakdown was different between rural and metropolitan areas, with 8.25% vs 0.00% low-income, 85.57% vs 63.68% medium-income, and 6.19% vs 36.32% high-income, respectively (< 0.001 for all). There was a greater proportion of white patients in rural areas (86.60% vs 60.44%, p < 0.001), but less Hispanic patients (10.31% vs 27.89%, p < 0.001) and patients of other races (3.09% vs 8.95%, p=0.049). There were no significant differences between metropolitan and non-metropolitan counties with respect to proportion male, age, and proportion of Black patients (Table 1).
Tumor Size
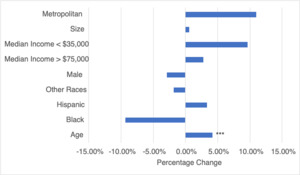
Metropolitan areas had a 9.50% smaller tumor size (p<0.001, CI:[-11.57%, -7.32%]), compared to non-metropolitan counties. Patients with Black race had a 14.32% larger tumor size (p<0.001, CI:[9.53%, 19.36%]), and male sex was associated with a 15.34% larger tumor size (p<0.001, CI:[13.66%, 17.12%]). A one-year increase in age was associated with a 1.55% increase in tumor size (p<0.001, CI:[13.66%, 17.12%]). Counties with a median income greater than $75,000 had 1.8% larger tumor size (p=0.02, CI:[0.20%, 3.46%]), and counties with median income less than $35,000 had a 15.70% smaller tumor size (p<0.001, CI:[-22.12%%, -8.80%]), compared to those from counties with 35-75K median income (Table 2, Figure 1).
Time to Treatment
A one-year increase in age and was associated with a 4.19% increase in time to treatment per year (p<0.001, CI:[2.43%, 5.97%]). All other co-variates were not found to be statistically significant, include living in a metropolitan county (Table 3, Figure 2).
Survival Analysis
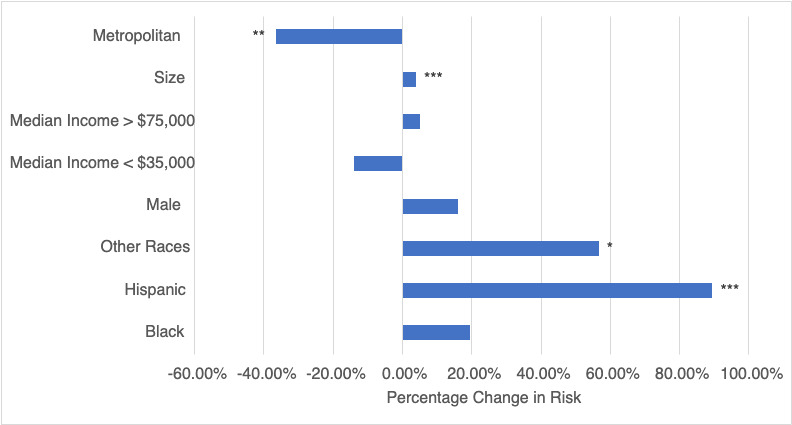
The Cox Proportional Hazard model estimated that the hazard ratio for cancer-specific mortality was 0.64 (p ≤ 0.04; 95% CI: [0.42, 0.97]) when comparing a patient with ES living in a metropolitan area versus a non-metropolitan area. Thus, metropolitan areas had a 36% lower risk of death over time. If the median patient in the dataset lived in a metropolitan area, the expected survival is 132.31 months. By comparison, if such a patient lived in a non-metropolitan area, the expected survival is 10 months shorter (112.68 months) (Table 4, Figure 3).
DISCUSSION
Ewing sarcoma is an aggressive cancer which requires specialist treatment.13 Prior oncology studies have revealed a disparity between rural and urban populations, including incidence and mortality, with rural populations suffering from worse outcomes.14,15 With oncological care predominantly centered around large academic centers, research has focused on understanding how rural or urban living affects delivery of care and the subsequent prognosis. One study previously revealed that 60% of rural Medicare patients attain care at the nearest available hospital, regardless of its size.16 The present study investigated the association between rurality and oncological outcomes while controlling for crucial socioeconomic factors. Metropolitan areas had a 36% lower risk of death over time, with the median patient in a non-Metropolitan region having a 10-month shorter survival.
Size of the tumor at time of diagnosis is another important factor to consider, with increasing size associated with incidence of metastatic disease.17 Ye et al. found that a tumor size greater than 8 cm had 2.55 increased odds of metastasis at time of diagnosis.18 In this study, metropolitan areas had a 9.50% smaller tumor size, as compared to non-metropolitan areas. The effect of geographic location may be explained by the access to treatment in metropolitan areas, with patients in rural areas having greater difficulty in attaining specialist care.19 Patients in rural locations face challenges including the likelihood of receiving specialized treatments and the lack of opportunities to participate in clinical trials.20 This is demonstrated by Lin et al. who revealed increased travel burden was associated with decreased likelihood of receiving chemotherapy in colon cancer patients.21 Additionally, Black patients also had an increased tumor size at the time of diagnosis. This finding corroborates with prior studies which reveal African Americans often present with larger tumors and do not undergo radiation therapy or surgical resection for soft-tissue sarcomas as frequently as Caucasian patients.22,23 Caucasians have the highest reported incidence of ES, as confirmed in the current study where White patients have the highest proportion of cases.24 Subsequently, the increased time to diagnosis may partly be explained by clinicians under-recognizing signs of ES in this patient group. Later diagnosis with increased tumor size at presentation may also be amplified by the absence of a national screening policy. Racial and geographic disparities must be further evaluated to ameliorate access to care for at-risk patient groups.
ES is an aggressive tumor, although improvements in oncological care have increased survival for localized tumors from 10% to 75%.25 Despite this, patients with metastatic ES continue to have a poor prognosis. An epidemiological study from the United Kingdom, found that for both osteosarcoma and ES, the distance from urban centers was associated with early mortality.25 Similarly, in the US cancer mortality is higher in rural compared to urban areas suggesting the critical role of geographic location on outcomes.26 The present study provides further evidence for this association as the median patient in a non-metropolitan region had a 10-month shorter survival compared to the metropolitan region. In addition to the availability and quality of care in urban areas, there are further reasons why this association may exist. Rural residents are more likely to have lower income, lower educational attainment, and higher unemployment.27 This is supported by the current study which found a greater proportion of patients in the metropolitan group had a higher income. These socioeconomic factors may all contribute to worse survival outcomes and further investigation should address how these disparities can be corrected. Yabroff et al. outlined community-level resources that may play a role, including availability of public transportation, access to health insurance coverage, and internet connection to facilitate telehealth consultations.26
The current investigation has provided further insight into geographic and socioeconomic factors which impact outcomes in ES. Even when adjusting for several demographic factors, rural patients have increased tumor size at time of diagnosis and worse survival rates. However, the present study has limitations, inherent to any large database study. Firstly, there is incomplete individual-level data collected which means barriers to treatment are estimated from county-level statistics. Furthermore, certain variables such as education and employment are lacking, and this can be important to consider when evaluating differences between rural and urban regions. Secondly, inaccuracies in source data can occur due to miscoding reported by the regional registries. Thirdly, the SEER database does not provide information on additional variables, such as extra-skeletal ES or insurance status, which may affect outcomes investigated.
CONCLUSION
Patients in rural areas who are diagnosed with ES have poorer outcomes compared to those who live in metropolitan areas. In this study analysis, there is no significant difference in time to treatment, although patients in metropolitan areas had a smaller tumor size at time of diagnosis with a favorable survival rate for cancer-specific mortality. Importantly, metropolitan areas had a 36% lower risk of death over time. Additionally, patients with black race have a larger tumor size and longer time to treatment when controlling for sex, age, median county income, and rurality. Further work is needed to examine socioeconomic determinants of health and promote interventions to reduce healthcare disparities for at-risk communities.
Conflict of interest statement
DA, VK, AH, JG, CLM have no disclosures to report. Eren O. Kuris reports the following: grants: Scoliosis Research Society; consulting fees: Seaspine, Spineart; committee roles; SRS, NASS. Alan H. Daniels reports the following: consulting fees: Stryker, Orthofix, Spineart, and Medtronic; fellowship support from Orthofix, Medtronic.
Funding statement
No funding
Ethical statement
Publicly available database of de-identified patient data. No IRB required.