Introduction
Lower back pain (LBP) has a lifetime prevalence of 80% in the United States population, and is the third largest United States healthcare expenditure, behind diabetes and ischemic heart disease.1,2 Specifically, discogenic back pain (DBP) is a subcategory of back pain defined as pain due to pathology of the disc. Despite the absence of radiographic evidence of disc herniation compressing the spinal column or nerves, the pain in DBP is multifactorial and mainly stems from the degeneration of the intervertebral disc but is also thought to be a result of biomechanical instability, localized inflammation, vertebral endplate pathology, and reinnervation of the area with nociceptive, unmyelinated nerve fibers.3
DBP is often referred to as discogenic low back pain (DLBP) due to the frequency of lumbar discs displaying radiographic evidence of degeneration when compared to cervical or thoracic discs. The most commonly affected level of disc degeneration in both men and women is the L4/5 level.4 Diagnosis of DBP is based on provocative discography or CT discography.5,6 Treatment options for DBP range from conservative management to more invasive techniques.5 In this review, we aim to focus on the pathophysiology, presentation, diagnosis of DBP with a special emphasis on treatment.
Pathophysiology
DBP primarily results after years of pathologic disc degeneration. Within DBP, there are two types of degeneration, a physiologic degeneration of the disc with absence of back pain, and a pathologic form of disc degeneration known as degenerative disc disease (DDD). It was previously was thought that years of repetitive movement and weight bearing were the main cause of DDD; however, a historical cohort study comparing competitive athletes to control subjects demonstrated greater disc degeneration in the lumbar spine of athletes; interestingly, there was less associated back pain in the athlete group than the control group.7 Twin studies have revealed primary contributing factors to disc degeneration such genetics and the patient’s body mass index.8 Additionally, environmental factors such as smoking, aging, and injury have been found to contribute and cause disc degeneration.3 Ultimately, there is a shift towards cell senescence and/or catabolic activity initiating a cascade of events caused by a deficiency of nutrients in the vascularized vertebral endplates leading to a decrease in disc height from loss of hyaluronic acid and water content from the nucleus pulposus and the deposition of collagen fiber.6,9 These physiologic changes cause cytokine release, inflammation, and microscopic tearing of the annulus fibrosus. The inflammatory cytokine release and tissue damage leads to neovascularization and neoinnervation through the annulus fibrosus and into the nucleus pulposus.
The pathophysiology of disc degeneration in DBP can be thought about as occurring in three steps. The first step is the initiation of genomic instability and concomitant extracellular membrane degradation of the disc.10 The second step is the body’s response to the damage.10 When the disc has undergone extrinsic or intrinsic damage, there are subsequent changes in cellular homeostasis, including dysregulated pathway signaling (MAPK and NF-κB), which in turn can lead to apoptosis or cell growth arrest.10
The third and final step can be categorized as loss of disc structure and physiologic function, which can be seen on radiography with loss of disc height and as a hypointensity within the disc on T2 weighted MRI suggesting water and proteoglycan loss within the nucleus pulposus.10 Though the loss of disc height and hypointensity on T2 weighted MRI can be seen in normal disc aging, it should be considered pathologic disc degeneration when there is neovascularization and neoinnervation occuring.3 Pain will not always be present when there are radiographic signs of disc degeneration; however, when pain is associated with acute and chronic disc degeneration, it is considered DBP. Of note, there is no radiographic herniation of the nucleus pulposus through the annulus fibrosus with DBP.11
Epidemiology and Risk Factors
In the United States alone, it is estimated that more than $90 billion dollars is spent annually on the diagnosis and treatment of LBP.12,13 In 2015, the Global Spine Care Initiative reported LBP to be the fourth leading cause of disability-adjusted life years (DALYs) globally.14 It is estimated that one in three people report having LBP, and the lifetime prevalence ranges from 60-80% of the population.15 Adding to the existing burden, the prevalence of LBP has been growing exponentially over the past few decades.14
LBP is caused by a variety of conditions; however, DBP is the most common cause of chronic LBP (CLBP) in both the young and elderly.14–17 LBP attributable to DBP has been estimated to be the cause of 26-42% of all LBP cases.16,18 The variance in this statistic is likely due to the difficulty of diagnosis. Accurate diagnosis is hindered due to the invasiveness of discography, and the concern that it may provoke pain in asymptomatic patients without much benefit. However, the benefit of lumbar discography is that in the case of symptomatic patients, it has a relatively low false positive rate for discogenic pain.19,20
DBP is pain secondary to disc degeneration without herniation or disc protrusion.15,21,22 Therefore, the risk factors for DBP parallels the risk factors of disc degeneration.18,21,23 The early disc degeneration that is associated with DBP arises from a combination of risk factors from anatomic, genetic, and environmental origins.23 Specific risk factors for DBP include advanced age, male gender, mechanical loading from trauma, obesity or occupational exposure, smoking, inflammation or infection such as occult discitis or autoimmune discitis, metabolic disorders such as diabetes mellitus, reduced cellularity, and conditions with poor healing.15,21,23–25 Genetic factors such as the presence of increased levels of cytokine IL1A or MMP13 have also been associated with early disc degeneration and the resultant DBP.23,25
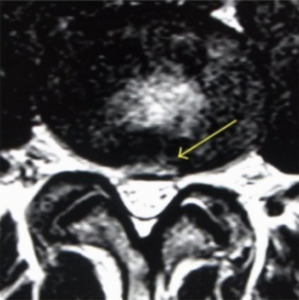
Since not all disc degeneration leads to DBP, efforts have been made to identify radiographic findings that predict progression to discogenic pain and aid in diagnosis.19,25–27 One such finding is the presence of Modic changes on MRI, which represent vertebral bone marrow lesions. Modic changes have been demonstrated to have a high specificity for DBP.25 High-intensity zones (HIZ) on MRI have also been identified as a risk factor for DBP, although with less sensitive and specific (Figure 1).26 Additionally, CT with provocative contrast discography has demonstrated a relationship between discogenic pain and annular tears.19
Presentation and Diagnosis
DBP is a specific and localizable form of LBP.15,24,27 Pain is often provoked with forward flexion of the torso while leaning forward or sitting down, as well as during coughing or sneezing. Flexion is provocative for DBP because it places more compressive stress on the intervertebral disc, and the pain can be worsened with increased axial loading or sitting.28 Pain may be alleviated by extension while standing up or ambulating.17,23,24 DBP primarily affects the L5/S1 and L4/L5 discs, respectively.29 Dermatomal radiation and other neurologic symptoms such as motor weakness, numbness, or incontinence are expected to be absent in DBP as these symptoms are evidence of advanced disc disease involving herniation, nerve compression, or spinal stenosis.15,17,18,23,30,31 Patients with DBP may report radiation to the buttocks and posterior thighs; however, radiation is not expected to pass below the knees.23,31
While the vast majority of DBP affects the lumbar region, it may also affect the thoracic and cervical spine.14,25,32 In cases of cervical DBP, dizziness has been reported as a presenting symptom. The neural hypersensitization and cytokine release associated with disc degeneration may alter proprioceptive inputs to the vestibular system and cause dizziness.32
In DBP, pain is in the mid axis of the spine with lateral movement.28 This centralization phenomenon can be used to differentiate pain originating in the disc from other cause of low back pain. Sensitivity has been reported to be 46-64% and specificity between 70-100%.28,33 Its utility as a diagnostic tool for discogenic pain; however, is limited due to lack of standardization and low sensitivity.28 Bone vibration test is a quick method to screen for DBP by applying vibratory stimuli to spinous process of affected vertebrae triggering a pain response. As a standalone tool, it has moderate sensitivity and specificity but improves when combined with other modalities such as MRI.28
MRI is useful for diagnosing DBP with particular attention to HIZ, nuclear signal, disc height, disc contour, and bone marrow intensity change.33,34 Disc degeneration on T2 weighted MRI appears as hypointense discs due to decreased signal intensity.28 HIZ on MRI are areas of hypodensity located on posterior annulus fibrosus in lumbar scans. It is theorized that inflammation of the annular fibrosus fissure causes the HIZ to appear and is the source of pain in DBP (Figure 1). One study found that TNF-alpha and CD-68 positive cells were higher in number in HIZ than in controls.35 However, HIZ, has good sensitivity but low specificity for DBP.36 Modic changes on MRI aid in the diagnosis of DBP as intensity changes on T2 and T1 weighted sequences are used to classify scans of vertebral endplates and subchondral bone into categories describing inflammatory changes, fatty degeneration of bone marrow, and sclerosis of subchondral bone (Figure 2).36 The combination of different MRI parameters increases its utility as a diagnostic tool.37
Provocative discography is an invasive diagnostic procedure in which contrast is injected into nucleus pulposus of a painful disc under pressurized conditions to induce pain. Performing discography at the level of pain may reproduce the patient’s pain and serve as a sensitive and specific diagnostic tool. Pain reproduction is attributed to contrast extravasation into annular tears or defects in the endplate associated with disc degeneration and DBP.18,27 A positive discogram requires reproduction of pain > 6/10 in intensity and at a pressure < 15 pounds per square inch above opening pressure and volume less than 3.0 mL of contrast.28 This has a high sensitivity and specificity for the diagnosis of DBP. Disadvantages are the invasiveness and a false positive rate of 10%.38 Of note, patients are at increased risk of accelerated disc degeneration and disc herniation; thus, limiting its use to mainly patients who are planning a surgical procedure to confirm the diagnosis.39 Discography is safe if specified standards and guidelines are adhered.40
Differential Diagnosis
LBP is common with a reported lifetime prevalence of 80% with a 30% risk of conversion from acute to a chronic back pain.33 Diagnosing DBP can be challenging as signs and features overlap with other causes of back pain. In the initial evaluation for back pain, systemic causes and red flag symptoms such as malignancy, infection and trauma should be ruled out.41,42 DBP is marked by axial pain that can in rare occasions radiate to the buttocks, flank and thigh and exacerbated with increased axial load and sitting.43
Differentiating DBP from a closely related cause of LBP, vertebrogenic back pain (VBP), can be difficult due to the proximity and overlapping symptoms.44,45 In VBP, the pain transmission signals are carried via the basivertebral nerve originating from the sinuvertebral nerve, and in DBP, the signals are transmitted via the sinuvertebral nerve.44,45 VBP ultimately is due to the degeneration of the highly innervated vertebral endplate.44,45 When the endplates are damaged, proinflammatory mediators and neurogenic growth factors are released promoting proliferation of the basivertebral nerves leading to an increased sensitivity to pain.44–46 VPB typically presents with axial LBP that can be characterized as deep, burning, and aching in quality; the pain is often worsened with sitting, rising from a seated position, and bending forward at the waist.44,45 Physical exam focuses on eliciting the pain via flexing movements that place stress on the anterior column with possible tenderness on percussion at the affected vertebral level.44,45 Diagnosis can be confirmed via MRI which will demonstrate type 1 and 2 Modic changes, with or without endplate damage, and active Schmori nodes.44,45
Other causes of low back pain such as facet arthropathy, paraspinal muscle strain, and spondylolisthesis can present similarly while the presence of radicular symptoms could be mistaken for sacroiliac joint pain and piriformis syndrome. Careful history taking, physical exam, imaging, and laboratory tests can help to make diagnose of DBP. Pain provocation with Valsalva maneuvers, centralization of pain, and pain relief with standing differentiates discogenic pain from other causes of lower back pain.47 Hallmark of discogenic pain on imaging is intervertebral disc degeneration and the absence of disc herniation. Biomarkers have the potential to act as screening tools to differentiate discogenic pain from other causes of back pain because inflammatory markers and cytokines are elevated in DBP but not in LBP with sciatica pain.48
Treatment
Treatment of DBP involves a multifaceted approach with an emphasis on conservative measures including behavioral modification, pharmacologic management, and other non-pharmacologic interventions with invasive therapy reserved for select patients. Unfortunately, there is a paucity of data concerning the management of DBP; thus, treatment recommendations are often based on data for management of chronic nonspecific back pain.
Pharmacologic
Pharmacologic management of discogenic pain relies on the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen with additional agents used as adjuncts. A randomized controlled trial (RCT) that examined 50 patients with CLBP compared 200 mg celecoxib twice daily to 500 mg acetaminophen twice daily and found that celecoxib was significantly more effective for management of symptoms (p < 0.05).49 A multi-center double-blind RCT investigated the role of 60 mg intravenous methylprednisolone, 200 mg intravenous ketoprofen daily, and placebo in addition to a standard oral pain regimen (acetaminophen, nefopam, tramadol, and morphine) in 54 hospitalized patients with acute discogenic sciatica.50 There was no significant improvement in leg pain across the 3 study arms; however, there was a statistically significant clinical response in leg pain on day 3 of methylprednisolone compared to ketoprofen or placebo.50
Gabapentin is a commonly prescribed adjunct for management of DBP. A double-blind RCT compared gabapentin versus placebo in 108 subjects with CLBP over a 12-week period.51 Participants in the intervention group were titrated to a target dose of 3,600 mg of gabapentin daily or the maximum tolerated dose.51 Although pain intensity was reduced by approximately 30% from baseline in all participants, there was no significant difference between pain reduction in the intervention and control group; however, this data is limited by a small sample size and high attrition rate.51
Muscle relaxants are another commonly used adjunct for DBP. A meta-analysis of 31 RCTs found that short courses of non-benzodiazepine antispasmodics for acute LBP yielded a significant reduction in pain compared to the placebo; however, it did not find improvement in disability and may be associated with an increase in adverse events.52 Moreover, antidepressants may be another effective adjunct for the management of DBP. A systematic review that examined data from 5 RCTs on the effectiveness of duloxetine demonstrated that duloxetine was an effective treatment for management of CLBP with 60 mg daily as the recommended dose based on efficacy and adverse event profile.53 A double-blind RCT studied the effectiveness of tricyclic antidepressants on management of CLBP compared low-dose amitriptyline (25 mg daily) to benztropine (1 mg daily) in 146 participants with CLBP.54 There was not a statistically significantly reduction in pain at 3 or 6 months.54 However, there was a statistically significant improvement in disability in the amitriptyline group at 3 months and a reduction in pain intensity at 6 months that was not significant, suggesting that amitriptyline may be a reasonable treatment option in select patients.54
Non-pharmacologic
While medical therapy with anti-inflammatory medications remain the mainstay of symptom alleviation, physical therapy should be incorporated into DBP treatment regimens, with emphasis placed on strengthening and stabilization of pelvic and core muscle groups.15–18,21,23,30,55 A meta-analysis of 249 RCTs found that exercise therapy is significantly more effective for management of CLBP compared to no treatment, usual care, or placebo.56 Furthermore, cognitive behavioral therapy (CBT) may be beneficial in the treatment of DBP. A meta-analysis of 22 RCTs evaluated the efficacy of CBT for treatment of CLBP comparing CBT versus control (i.e., usual care, physical therapy, and drug therapy) and found that patients treated with CBT had a statistically significant improvement in regard to disability, fear avoidance, and self-efficacy after intervention; however, there was no significant improvement at 3-, 6-, or 12-month follow-up. Subgroup analysis suggested that CBT was most effective when used in combination with other interventions.57
Moreover, acupuncture can be used as an adjunct for DBP. One RCT that evaluated acupuncture versus sham acupuncture across 12 sessions over 4 weeks in 46 participants with discogenic sciatica found improvement in weekly mean visual analog scale (VAS) scores from baseline for leg pain in the intervention group.58 Secondary outcomes including change from baseline mean VAS for leg pain or lower back pain, Oswestry Disability Index (ODI) scores, and 36-Item Short Form Health Survey (SF-36) scores were not statistically significant.58
Minimally Invasive
A recent meta-analysis of 7 RCTs including 655 participants found that transcutaneous electrical nerve stimulation (TENS) or interferential current (IFC) significantly improved pain intensity and reduced disability compared to placebo or control in individuals with CLBP and/or neck pain (CNP) during treatment but not immediately after or at 1- or 3- month follow-up.59 Another study of 20 patients found that dorsal root ganglion stimulation improved pain and quality of life at 12-month follow-up and disability at 6- and 12-month follow-up in patients with DBP.60
Spinal injections are often used for DBP that do not respond to conservative measures. A meta-analysis of 15 RCTs demonstrated moderate level evidence that epidural injections with lidocaine was effective for short- and long-term pain relief and improvement of functionality in patients with spinal pain; however, there was not a significant difference between injections with or without steroids.61 A meta-analysis of 3 RCTs found that methylene blue disc injections do significantly improve pain at 3- and 6-month follow-up and disability at 4-6 week and 3-month follow-up compared to control.62
An alternative option for the management of DBP is regenerative medicine. While regenerative therapies such as biologics and stem cell therapies hold much promise, outcomes in clinical trials have yet to demonstrate meaningful results.15,17,21–23,63 Interestingly, there does seem to be an age-dependent response to allogenic stem-cell therapy for the treatment of DBP.63 A meta-analysis of 21 studies found evidence that injection of medicinal signaling cells or mesenchymal stem cells and platelet-rich plasma may have a beneficial role in management of LBP; however, further RCTs are needed to fully evaluate their efficacy.64 The use of viable allogeneic disc tissue derived from cadavers has demonstrated promising results in young patients (< 42 years of age) who are afflicted with DBP.65,66 Unfortunately, in older patients (> 42 years of age) there was no difference between the control and allogeneic supplementation group plus.65 The study was limited by the use of saline injection as the control as it can elicit a clinical response.65,66 Fortunately, saline does not retain water due to the absence of proteoglycans; thus, patients were crossed over to the treatment group and had positive results not requiring retreatment at 36-month follow-up.65,66
Invasive
Data regarding efficacy of lumbar spinal fusion for CLBP is mixed. A meta-analysis of 6 studies comparing lumbar fusion versus nonoperative management for CLBP secondary to degenerative joint disease did not find a significant improvement in 24-month follow-up or disability both short- or long-term.67 Conversely, a meta-analysis of 25 prospective cohort studies including 1,777 participants with lumbar degenerative disorders who underwent first-time lumbar spinal fusion found significant improvement in back pain, leg pain, and disability at 24-month follow-up compared to pre-surgery.68
Both standard open discectomy and minimally invasive discectomy are options for treatment of DBP. A prospective study that evaluated the efficacy of total lumbar disc replacement in 201 patients found significant improvement in pain and disability at 3-, 6-, and 12-month follow-up compared to baseline; however, there was a significant worsening in pain scores after 48-month follow-up.69 The role of minimally invasive discectomy was investigated in a study with 300 patients with DBP secondary to single-level herniated disc which revealed a significant improvement of symptoms based on VAS and modified Suezawa and Schreiber clinical scoring system (MSS) scores.70 Finally, a retrospective study which evaluated 89 patients who underwent percutaneous endoscopic lumbar annuloplasty and nucleoplasty (PELAN) for DBP demonstrated improved outcomes based on the numeric rating scale, Oswestry disability index percent, and modified Macnab criteria at both short-term and long-term follow-up.71
Despite treatment, DBP is a chronic condition which can be resistant to long-term amelioration.22,30,72 Over 68% of patients with DBP reported ongoing pain despite non-surgical treatment; nevertheless, the progression to more serious conditions such as disc herniation, nerve root compression, or spinal stenosis in DBP was low at the 4-year follow-up period.72
Conclusion
Healthcare spending in the United States on LBP is upwards of $87.6 billion per year, making it the third most expensive disease, behind diabetes and ischemic heart disease.2 DBP, a subcategory of LBP, is an often difficult to diagnosis with treatments having limited efficacy. Further research studies are needed to better define which patient population would benefit from the different types of treatment. Furthermore, additional studies are needed to fully appreciate novel options such as regenerative medicine and viable allogeneic disc tissue which may be an alternative treatment avenue for select groups of patients.
Author Contributions
All authors were involved in the writing and editing of the manuscript.
Disclosures
There are no conflict of interests with the authors.